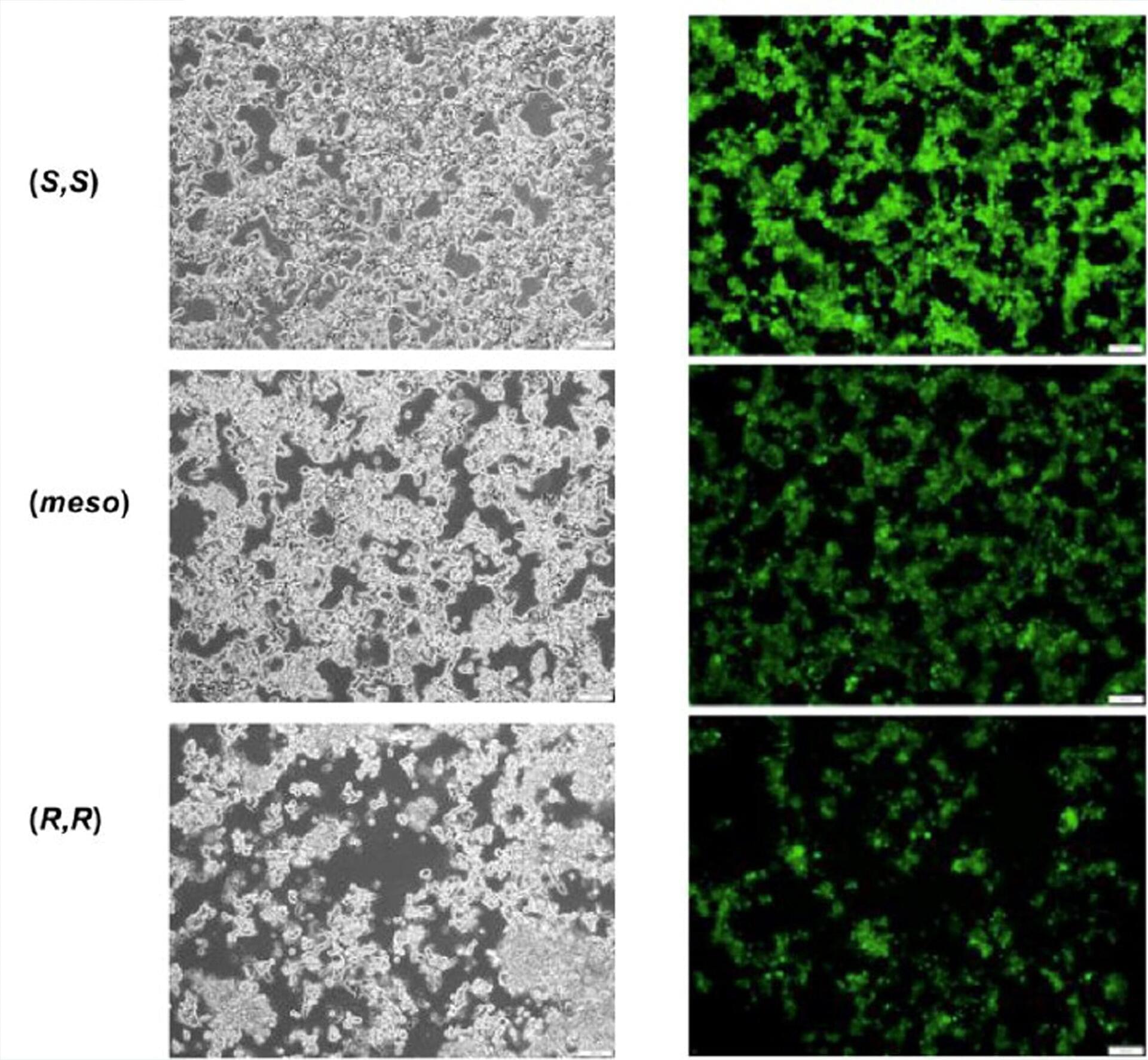
Scientific breakthroughs in one disease don’t always shed light on treating other diseases. But that’s been the surprising journey of one Mayo Clinic research team. After identifying a sugar molecule that cancer cells use on their surfaces to hide from the immune system, the researchers have found the same molecule may eventually help in the treatment of type 1 diabetes, once known as juvenile diabetes.
Type 1 diabetes is a chronic autoimmune condition in which the immune system errantly attacks pancreatic beta cells that produce insulin. The disease is caused by genetic and other factors and affects an estimated 1.3 million people in the U.S.
In their studies, the Mayo Clinic researchers took a cancer mechanism and turned it on its head. Cancer cells use a variety of methods to evade immune response, including coating themselves in a sugar molecule known as sialic acid. The researchers found in a preclinical model of type 1 diabetes that it’s possible to dress up beta cells with the same sugar molecule, enabling the immune system to tolerate the cells.