A mutation previously linked to skin disorders like psoriasis may also play a surprising role in gut health, according to new research published by scientists at VIB-UGent and colleagues from UGent, the University of Barcelona, and University College London. This mutation activates skin immune responses but also affects the intestine.
This finding, published in EMBO Molecular Medicine, reveals a new connection between genetics, the immune system, and the gut, which may have therapeutic implications.
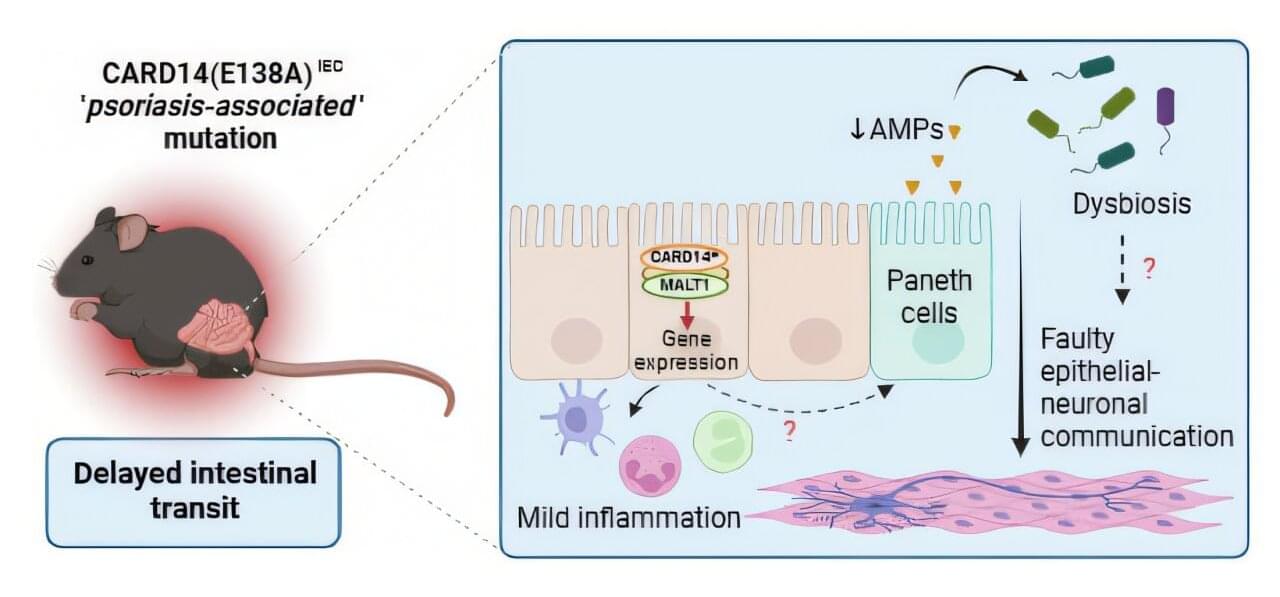
Scientists under the leadership of Dr. Inna Afonina and Prof. Rudi Beyaert (VIB-UGent Center for Inflammation Research) have found that a mutation in the gene CARD14, known for activating skin immune responses in psoriasis patients, also affects the intestine. This mutation reduces gut motility, promotes mild inflammation, and increases vulnerability to bacterial infections.