BECOMING a parent for the first time is a major moment for anyone — but for Laura Gayton, giving birth to a healthy, crying baby boy…


The recently identified, globally predominant SARS-CoV-2 Omicron variant (BA.1) is highly transmissible, even in fully vaccinated individuals, and causes attenuated disease compared with other major viral variants recognized to date1 – 7. The Omicron spike (S) protein, with an unusually large number of mutations, is considered the major driver of these phenotypes3,8. We generated chimeric recombinant SARS-CoV-2 encoding the S gene of Omicron in the backbone of an ancestral SARS-CoV-2 isolate and compared this virus with the naturally circulating Omicron variant. The Omicron S-bearing virus robustly escapes vaccine-induced humoral immunity, mainly due to mutations in the receptor-binding motif (RBM), yet unlike naturally occurring Omicron, efficiently replicates in cell lines and primary-like distal lung cells. In K18-hACE2 mice, while Omicron causes mild, non-fatal infection, the Omicron S-carrying virus inflicts severe disease with a mortality rate of 80%. This indicates that while the vaccine escape of Omicron is defined by mutations in S, major determinants of viral pathogenicity reside outside of S.
The authors have declared no competing interest.
Cleveland Clinic researchers have identified a common diabetes medication, metformin, as a possible treatment for atrial fibrillation.
The study, published in Cell Reports Medicine, built on ongoing collaborative Cleveland Clinic research to support further investigation into metformin as a drug repurposing candidate. Researchers used advanced computation and genetic sequencing to determine that metformin’s targets overlap significantly with genes dysregulated in atrial fibrillation.
Finding drugs or procedures to treat atrial fibrillation is difficult because of potential serious side effects. There is a significant need for new treatments for atrial fibrillation as there have been no new drugs approved in more than a decade.
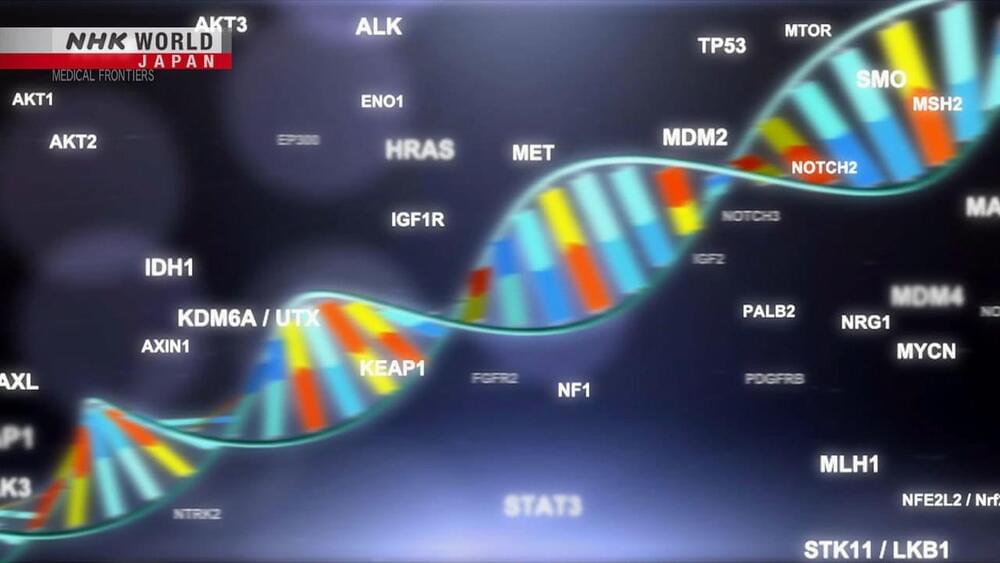
Genomic medicine is undergoing rapid change after the Japanese public health insurance system began to cover genetic testing in 2019. Cancer patients who meet certain criteria are able to take these tests for a relatively affordable price, and their genetic information is collected in a massive database and analyzed with the help of around 170 hospitals across the country. But challenges remain, with suitable drugs available for only 10% of patients who undergo testing.

Quantum effects play a hitherto unexpected role in creating instabilities in DNA – the so-called “molecule of life” that provides instructions for cellular processes in all living organisms. This conclusion, based on work by researchers at the University of Surrey in the UK, goes against long-held beliefs that quantum behaviour is not relevant in the wet, warm environment of cells, and could have far-reaching consequences for models of genetic mutation.
The two strands of the DNA double helix are linked together by hydrogen bonds between the DNA bases. There are typically four different bases, called Guanine (G), Cytosine ©, Adenine (A) and Thymine (T). In the standard configuration, A always bonds to T while C always bonds to G. However, if the protons (nuclei of the hydrogen atoms) that make up the bonds hop from one strand of DNA to the other then a genetic mutation can occur.
\r \r
*Intelligence Beyond the Brain: morphogenesis as an example of the scaling of basal cognition*
*Description:*
Each of us takes the remarkable journey from physics to mind: we start life as a quiescent oocyte (collection of chemical reactions) and slowly change and acquire an advanced, centralized mind. How does unified complex cognition emerge from the collective intelligence of cells? In this talk, I will use morphogenesis to illustrate how evolution scales cognition across problem spaces. Embryos and regenerating organs produce very complex, robust anatomical structures and stop growth and remodeling when those structures are complete. One of the most remarkable things about morphogenesis is that it is not simply a feed-forward emergent process, but one that has massive plasticity: even when disrupted by manipulations such as damage or changing the sizes of cells, the system often manages to achieve its morphogenetic goal. How do cell collectives know what to build and when to stop? Constructing and repairing anatomies in novel circumstances is a remarkable example of the collective intelligence of a biological swarm. I propose that a multi-scale competency architecture is how evolution exploits physics to achieve robust machines that solve novel problems. I will describe what is known about developmental bioelectricity — a precursor to neurobiology which is used for cognitive binding in biological collectives, that scales their intelligence and the size of the goals they can pursue. I will also discuss the cognitive light cone model, and conclude with examples of synthetic living machines — a new biorobotics platform that uses some of these ideas to build novel primitive intelligences. I will end by speculating about ethics, engineering, and life in a future that integrates deeply across biological and synthetic agents.
As the 3rd presenter during the morning session of the American Society for Dermatologic Surgery Meeting, “Emerging Concepts,” Saranya Wyles, MD, PhD, assistant professor of dermatology, pharmacology, and regenerative medicine in the department of dermatology at the Mayo Clinic in Rochester, Minnesota, explored the hallmarks of skin aging, the root cause of aging and why it occurs, and regenerative medicine. Wyles first began with an explanation of how health care is evolving. In 21st-century health care, there has been a shift in how medical professionals think about medicine. Traditionally, the first approach was to fight diseases, such as cancer, inflammatory conditions, or autoimmune disorders. Now, the thought process is changing to a root cause approach with a curative option and how to rebuild health. Considering how to overcome the sequence of the different medications and treatments given to patients is rooted in regenerative medicine principles.
For skin aging, there is a molecular ‘clock’ that bodies follow. Within the clock are periods of genomic instability, telomere attrition, and epigenetic alterations, and Wyles’ lab focuses on cellular senescence.
“We’ve heard a lot at this conference about bio stimulators, aesthetics, and how we can stimulate our internal mechanisms of regeneration. Now, the opposite force of regeneration is the inhibitory aging hallmarks which include cellular senescence. So, what is cell senescence? This is a state that the cell goes into, similar to apoptosis or proliferation, where the cell goes into a cell cycle arrest so instead of dividing apoptosis, leading to cell death, the cell stays in this zombie state,” said Wyles.

Fellow webinar participant Christopher Mason, a geneticist at Weill Cornell Medicine, argued that gene-hacking astronauts could even be a moral imperative.
“And are we maybe ethically bound to do so?” Mason said. “I think if it’s a long enough mission, you might have to do something, assuming it’s safe, which we can’t say yet.”

After decades of exhaustive study, scientists have concluded that human tetrachromacy is real. Some people have a truly superhuman range of color vision. In fact, there are two distinct types of tetrachromacy. In some cases, it’s genetic. But in some rare cases, it can also be an acquired trait. While it’s difficult to test, enough tetrachromats have stepped forward that scientists now have visual and genetic tests for the condition.
One percent of the world’s population is thought to be tetrachromatic. These lucky folks may be able to see a thousand times as many colors as the rest of us trichromats. In order to test that idea, researcher Gabriele Johnson devised an experiment. She used precise amounts of pigment to create shades of paint that could only be distinguished by a machine — or a tetrachromat. In 2010, Johnson found a subject who was able to tell each subtle shade apart, every time — just as fast as trichromats could identify the colors they saw. “When you ask them to discriminate between the two mixtures, a tetrachromat can do it very quickly,” she said. “They don’t hesitate.”
Concetta Antico is a painter and art teacher with genetic tetrachromacy. Growing up in Sydney, she says, she was always “a little bit out of the box,” alone in her own visual dreamland. She always preferred the kaleidoscope of colors she saw when she looked at the natural world. But nobody else seemed to see it quite like she did. So she decided to paint what she saw. “I’m sure people just think I’m high on something all the time,” she said, “but I’m really just high on life and the beauty that’s around us.”

Neuroscience is contributing to an understanding of the biological bases of human intelligence differences. This work is principally being conducted along two empirical fronts: genetics—quantitative and molecular—and brain imaging. Quantitative genetic studies have established that there are additive genetic contributions to different aspects of cognitive ability—especially general intelligence—and how they change through the lifespan. Molecular genetic studies have yet to identify reliably reproducible contributions from individual genes. Structural and functional brain-imaging studies have identified differences in brain pathways, especially parieto-frontal pathways, that contribute to intelligence differences. There is also evidence that brain efficiency correlates positively with intelligence.