Exactly how is a person’s eye color determined? It depends on the amount, type and distribution of melanin, or pigment, in the iris of the eye, a genetics expert reveals — and more.


A University of Michigan-led study based on a review of genetic and health information from more than 276,000 people finds strong support for a decades-old evolutionary theory that sought to explain aging and senescence.
In 1957, evolutionary biologist George Williams proposed that genetic mutations that contribute to aging could be favored by natural selection if they are advantageous early in life in promoting earlier reproduction or the production of more offspring. Williams was an assistant professor at Michigan State University at the time.
Williams’ idea, now known as the antagonistic pleiotropy theory of aging, remains the prevailing evolutionary explanation of senescence, the process of becoming old or aging. While the theory is supported by individual case studies, it has lacked unambiguous genome-wide evidence.
Welcome to Lifespan with Dr. David Sinclair. Dr. David Sinclair is a professor of genetics and co-director of Harvard Medical School’s Paul F. Glenn Center for Biology of Aging Research.
Biologist and genetics expert Dr. David Sinclair is out to prove he can live past 100 years old, and he thinks you can too. On this episode Sinclair goes in-depth on the process of aging and the techniques you can incorporate into your life that help you live a longer, healthier life, including optimizing your diet, the benefits of exercise, the role of a positive attitude, the importance of sleep, the three supplements he takes every day, why it’s never too late to slow the process of aging, and so much more.
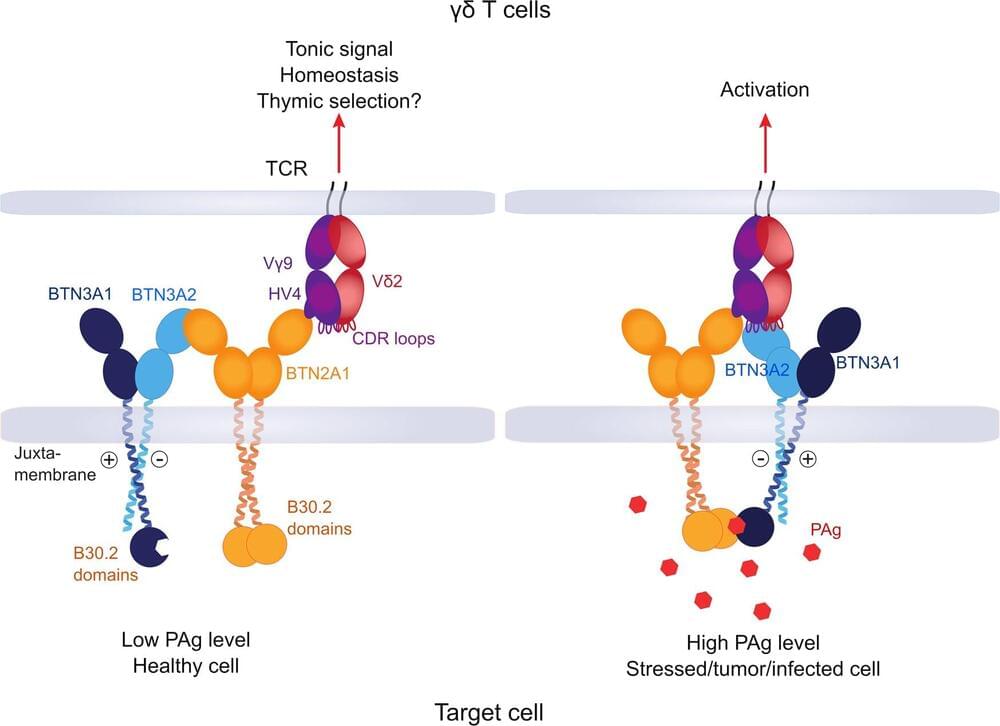
In order for immune cells to do their job, they need to know against whom they should direct their attack. Research teams at the University of Würzburg have identified new details in this process.
As complicated as their name is, they are important for the human organism in the fight against pathogens and cancer: Vγ9Vδ2 T cells are part of the immune system and, as a subgroup of white blood cells, fight tumor cells and cells infected with pathogens. They recognize their potential victims by their altered cell metabolism.
Research teams from the University of Würzburg and the University Hospital of Würzburg, together with groups in Hamburg, Freiburg, Great Britain and the U.S., have now gained new insights into how these cells manage to look inside the cell. Thomas Herrmann, Professor of Immunogenetics at the Institute of Virology and Immunobiology and his colleague Dr. Mohindar Karunakaran at Julius-Maximilians-Universität Würzburg (JMU), were responsible for the study published in the journal Nature Communications.
In this episode, Dr. David Sinclair and co-host Matthew LaPlante discuss why we age. In doing so, they discuss organisms that have extreme longevity, the genes that control aging (mTOR, AMPK, Sirtuins), the role of sirtuin proteins as epigenetic regulators of aging, the process of “ex-differentiation” in which cells begin to lose their identity, and how all of this makes up the “Information Theory of Aging”, and the difference between “biological age” and “chronological age” and how we can measure biological age through DNA methylation clocks. #Aging #DavidSinclair #Longevity

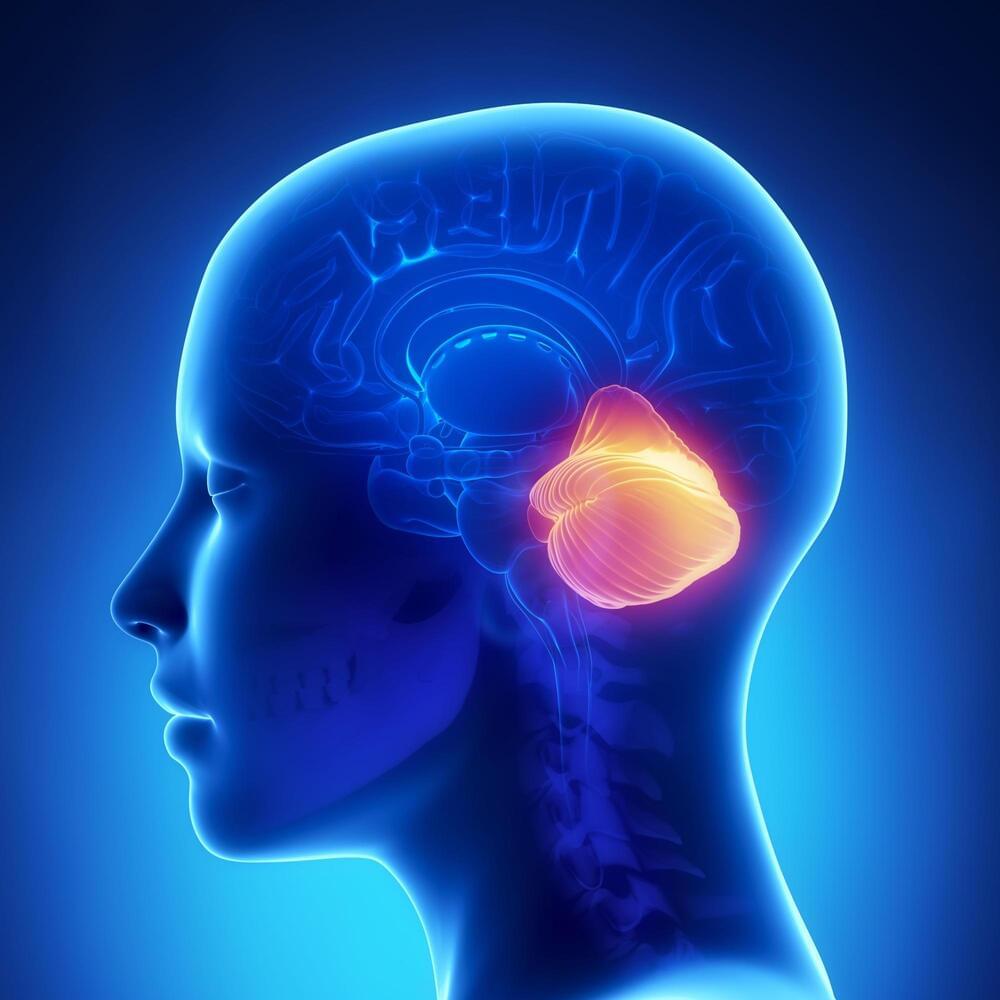
The advancement of higher cognitive abilities in humans is predominantly associated with the growth of the neocortex, a brain area key to conscious thinking, movement, and sensory perception. Researchers are increasingly realizing, however, that the “little brain” or cerebellum also expanded during evolution and probably contributes to the capacities unique to humans, explains Prof. Henrik Kaessmann from the Center for Molecular Biology of Heidelberg University.
His research team has – together with Prof. Dr Stefan Pfister from the Hopp Children’s Cancer Center Heidelberg – generated comprehensive genetic maps of the development of cells in the cerebella of humans, mice, and opossums. Comparisons of these data reveal both ancestral and species-specific cellular and molecular characteristics of cerebellum development spanning over 160 million years of mammalian evolution.
Genome and Structure:
HIV’s genome is a 9.7 kb linear positive-sense ssRNA.1 There is a m7G-cap (specifically the standard eukaryotic m7GpppG as added by the host’s enzymes) at the 5’ end of the genome and a poly-A tail at the 3’ end of the genome.2 The genome also has a 5’-LTR and 3’-LTR (long terminal repeats) that aid its integration into the host genome after reverse transcription, that facilitate HIV genetic regulation, and that play a variety of other important functional roles. In particular, it should be noted that the integrated 5’UTR contains the HIV promoter called U3.3,4
HIV’s genome translates three polyproteins (as well as several accessory proteins). The Gag polyprotein contains the HIV structural proteins. The Gag-Pol polyprotein contains (within its Pol component) the enzymes viral protease, reverse transcriptase, and integrase. The Gag-Pol polyprotein is produced via a −1 ribosomal frameshift at the end of Gag translation. Because of the lower efficiency of this frameshift, Gag-Pol is synthesized 20-fold less frequently than Gag.5 The frameshift’s mechanism depends upon a slippery heptanucleotide sequence UUUUUUA and a downstream RNA secondary structure called the frameshift stimulatory signal (FSS).6 This FSS controls the efficiency of the frameshift process.
An MIT study suggests 3D folding of the genome is key to cells’ ability to store and pass on “memories” of which genes they should express.
Every cell in the human body contains the same genetic instructions, encoded in its DNA. However, out of about 30,000 genes, each cell expresses only those genes that it needs to become a nerve cell, immune cell, or any of the other hundreds of cell types in the body.
Each cell’s fate is largely determined by chemical modifications to the proteins that decorate its DNA; these modification in turn control which genes get turned on or off. When cells copy their DNA to divide, however, they lose half of these modifications, leaving the question: How do cells maintain the memory of what kind of cell they are supposed to be?
A new MIT study proposes a theoretical model that helps explain how these memories are passed from generation to generation when cells divide. The research team suggests that within each cell’s nucleus, the 3D folding of its genome determines which parts of the genome will be marked by these chemical modifications. After a cell copies its DNA, the marks are partially lost, but the 3D folding allows the cell to easily restore the chemical marks needed to maintain its identity. And each time a cell divides, chemical marks allow a cell to restore its 3D folding of its genome. This way, by juggling the memory between 3D folding and the marks, the memory can be preserved over hundreds of cell divisions.