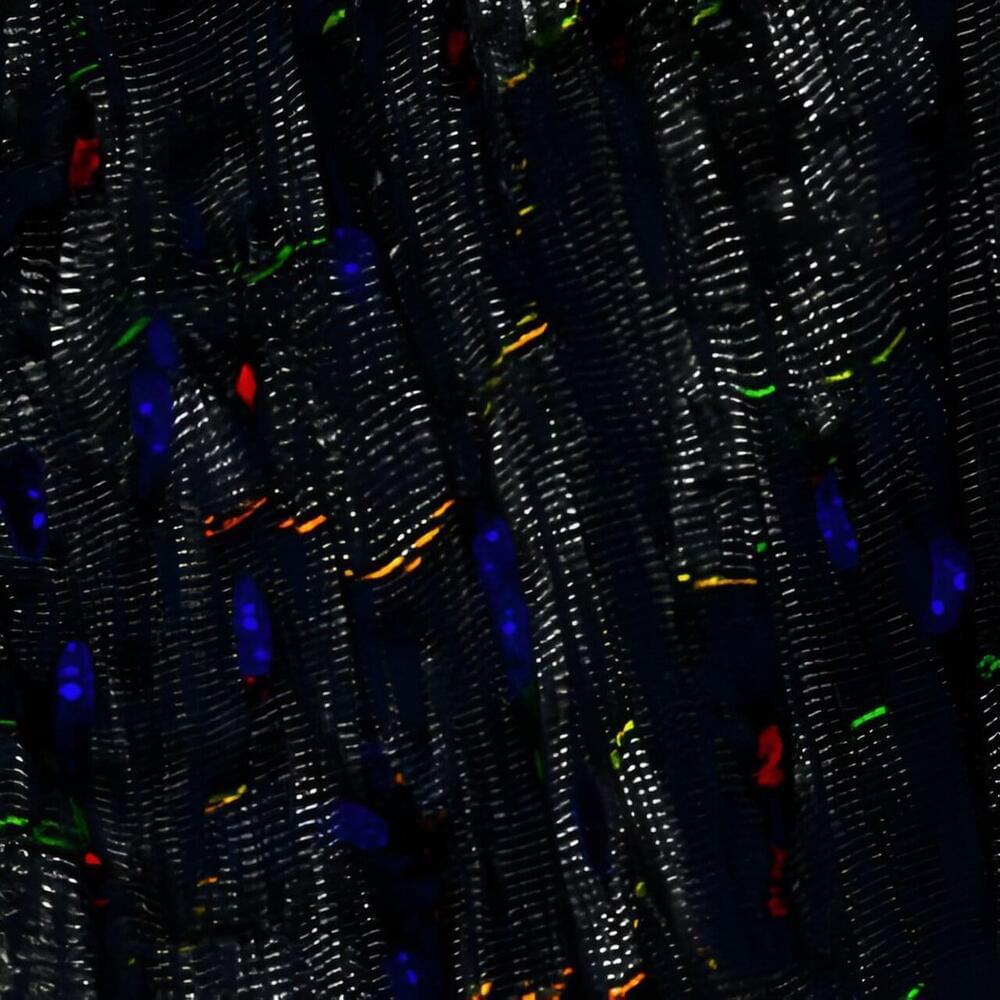
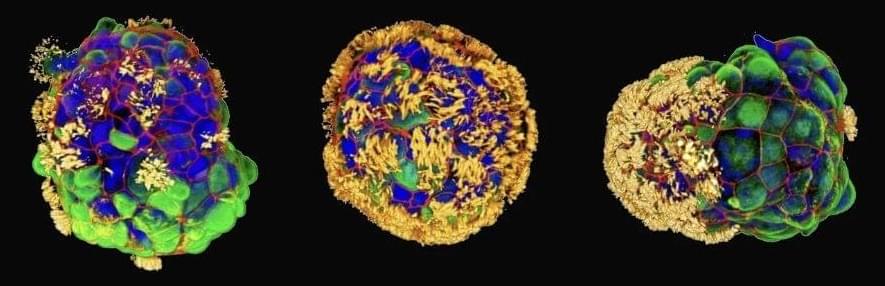
Researchers at the Hubrecht Institute have laid the foundation for the development of a gene therapy for the genetic heart disease arrhythmogenic cardiomyopathy (ACM). Their approach, based on replacement of the PKP2 gene, led to significant structural and functional improvements in laboratory models of the disease.
The study by the group of Eva van Rooij was published on 7 December 2023 in Nature Cardiovascular Research. Multiple clinical trials will start in 2024 in the United States to explore the clinical potential of this approach in ACM patients with PKP2 mutations.
ACM is a genetic heart disease that affects 1 in 2,000 to 1 in 5,000 people worldwide. It is characterized by arrhythmias and can lead to sudden cardiac arrest. Current treatment of the disease usually consists of antiarrhythmic drugs and implantable cardioverter-defibrillators (ICDs), which are focused solely on treating the symptoms rather than targeting the root of the problem.