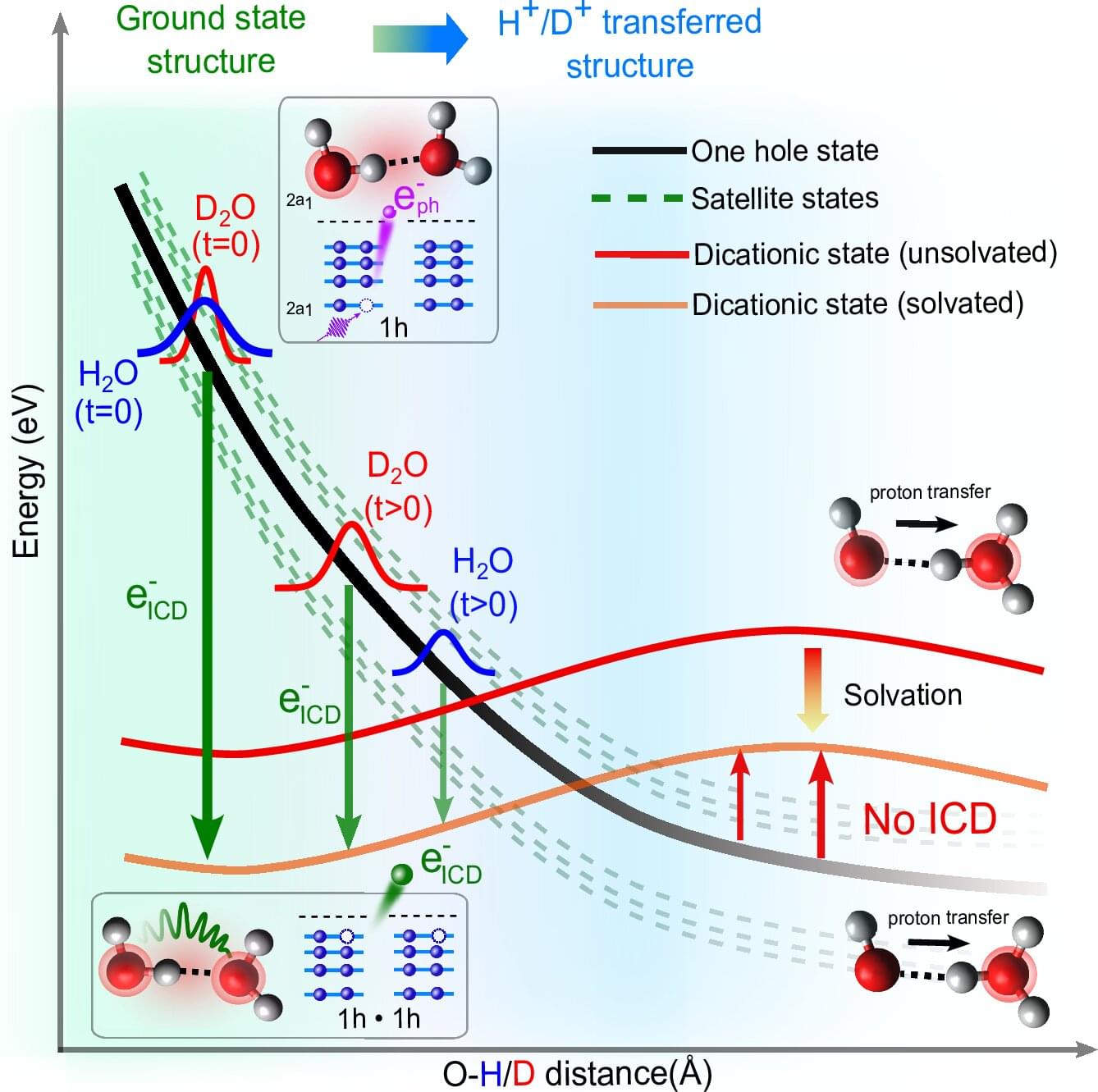
When high-energy radiation interacts with water in living organisms, it generates particles and slow-moving electrons that can subsequently damage critical molecules like DNA. Now, Professor Petr Slavíček and his bachelor’s student Jakub Dubský from UCT Prague (University of Chemistry and Technology, Prague) have described in detail one of the key mechanisms for the creation of these slow electrons in water, a process known as Intermolecular Coulombic Decay (ICD). Their powerful mathematical model successfully explains all the data from complex laser experiments conducted at ETH Zurich (Hans-Jakob Woerner team).
The work, which deepens the fundamental understanding of radiation chemistry, has been published in the journal Nature Communications.
A detailed knowledge of the processes in aqueous solutions, combined with advances in research technologies using high-energy radiation, is transforming the field of radiation chemistry. In the future, these insights could lead to significant changes in various fields, including medicine, particularly in developing more sensitive and controllable applications for devices based on ionizing radiation.