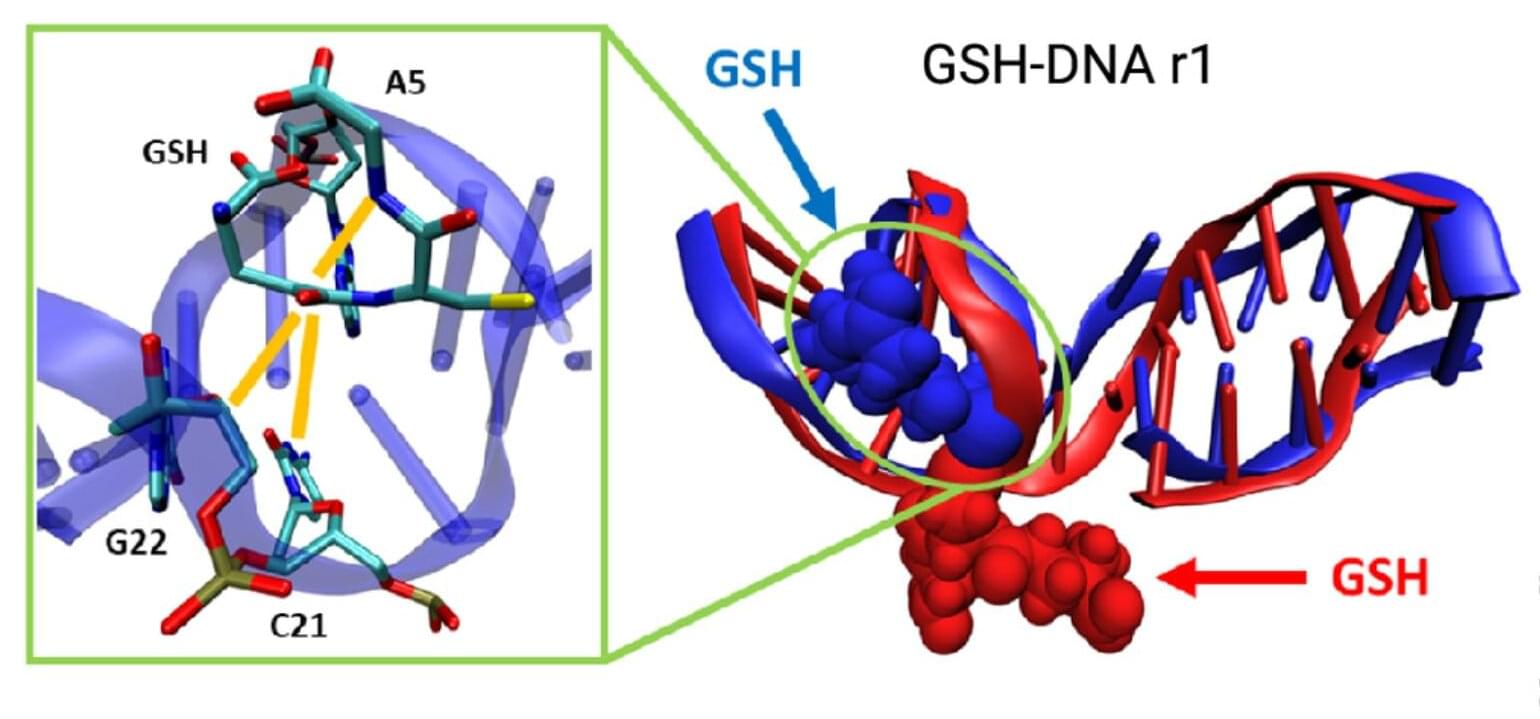
Researchers from McMaster University and the University of Pittsburgh have created the first functionally complete logic gate—a NAND gate (short for “NOT AND”)—in a soft material using only beams of visible light. The discovery, published in Nature Communications, marks a significant advance in the field of materials that compute, in which materials themselves process information without traditional electronic circuitry.
“This project has been part of my scientific journey for over a decade,” said first author Fariha Mahmood, who began studying the gels as an undergraduate researcher at McMaster and is now pursuing postdoctoral research at the University of Cambridge. “To see these materials not only respond to light but also perform a logic operation feels like watching the material ‘think.’ It opens the door to soft systems making decisions on their own.”
Mahmood is joined by authors Anna C. Balazs, distinguished professor of chemical and petroleum engineering, and Victor V. Yashin, research assistant professor at Pitt’s Swanson School of Engineering; and corresponding author Kalaichelvi Saravanamuttu, professor of chemistry and chemical biology at McMaster.