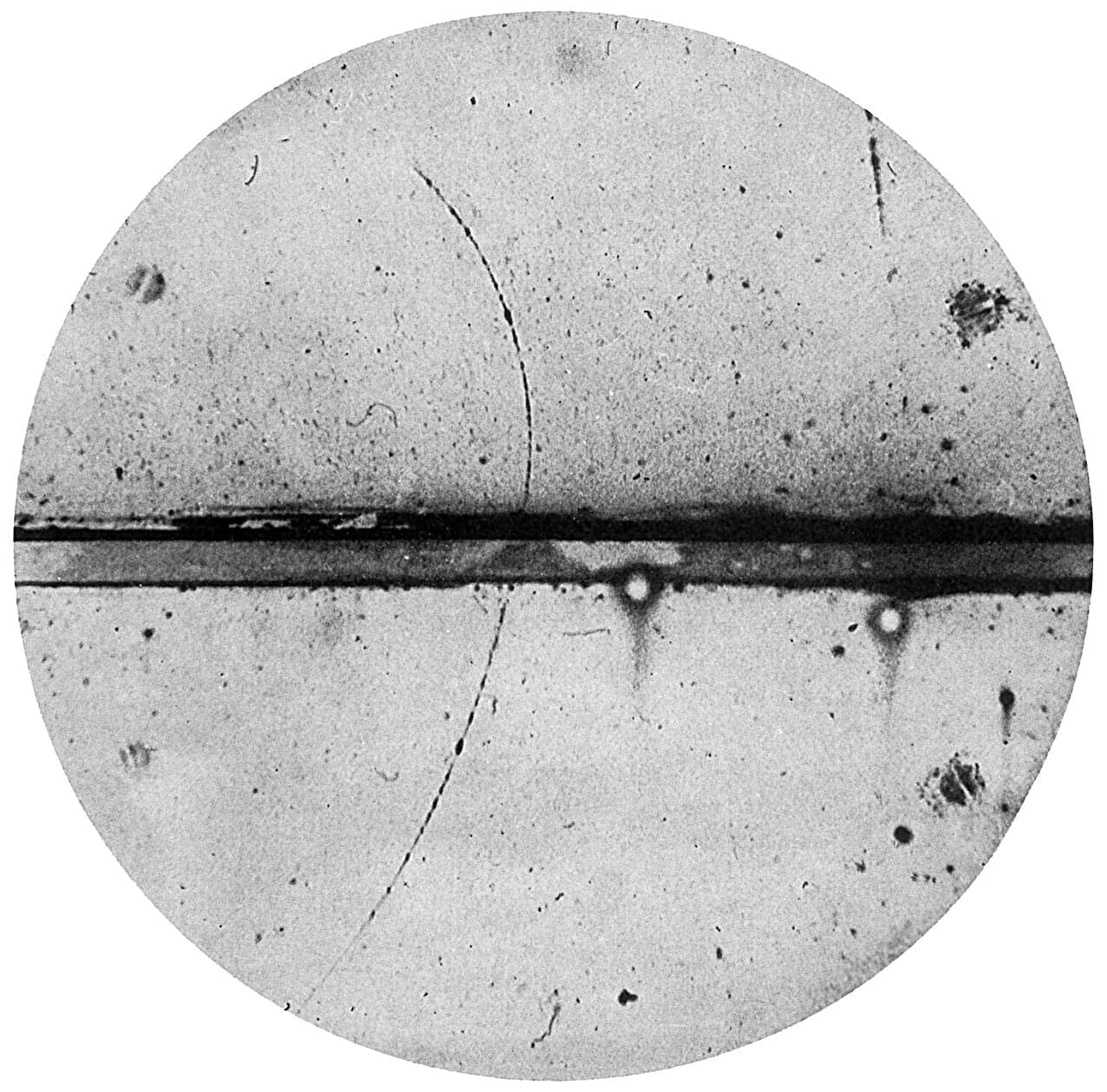
Chemical rockets have taken us to the moon and back, but traveling to the stars demands something more powerful. Space X’s Starship can lift extraordinary masses to orbit and send payloads throughout the solar system using its chemical rockets, but it cannot fly to nearby stars at 30% of light speed and land. For missions beyond our local region of space, we need something fundamentally more energetic than chemical combustion, and physics offers, or, in other words, antimatter.
When antimatter encounters ordinary matter, they annihilate completely, converting mass directly into energy according to Einstein’s equation E=mc². That c² term is approximately 10¹⁷, an almost incomprehensibly large number. This makes antimatter roughly 1,000 times more energetic than nuclear fission, the most powerful energy source currently in practical use.
As a source of energy, antimatter can potentially enable spacecraft to reach nearby stars at significant fractions of the speed of light. A detailed technical analysis by Casey Handmer, CEO of Terraform Industries, outlines how humanity could develop practical antimatter propulsion within existing spaceflight budgets, requiring breakthroughs in three critical areas; production efficiency, reliable storage systems, and engine designs that can safely harness the most energetic fuel physically possible.