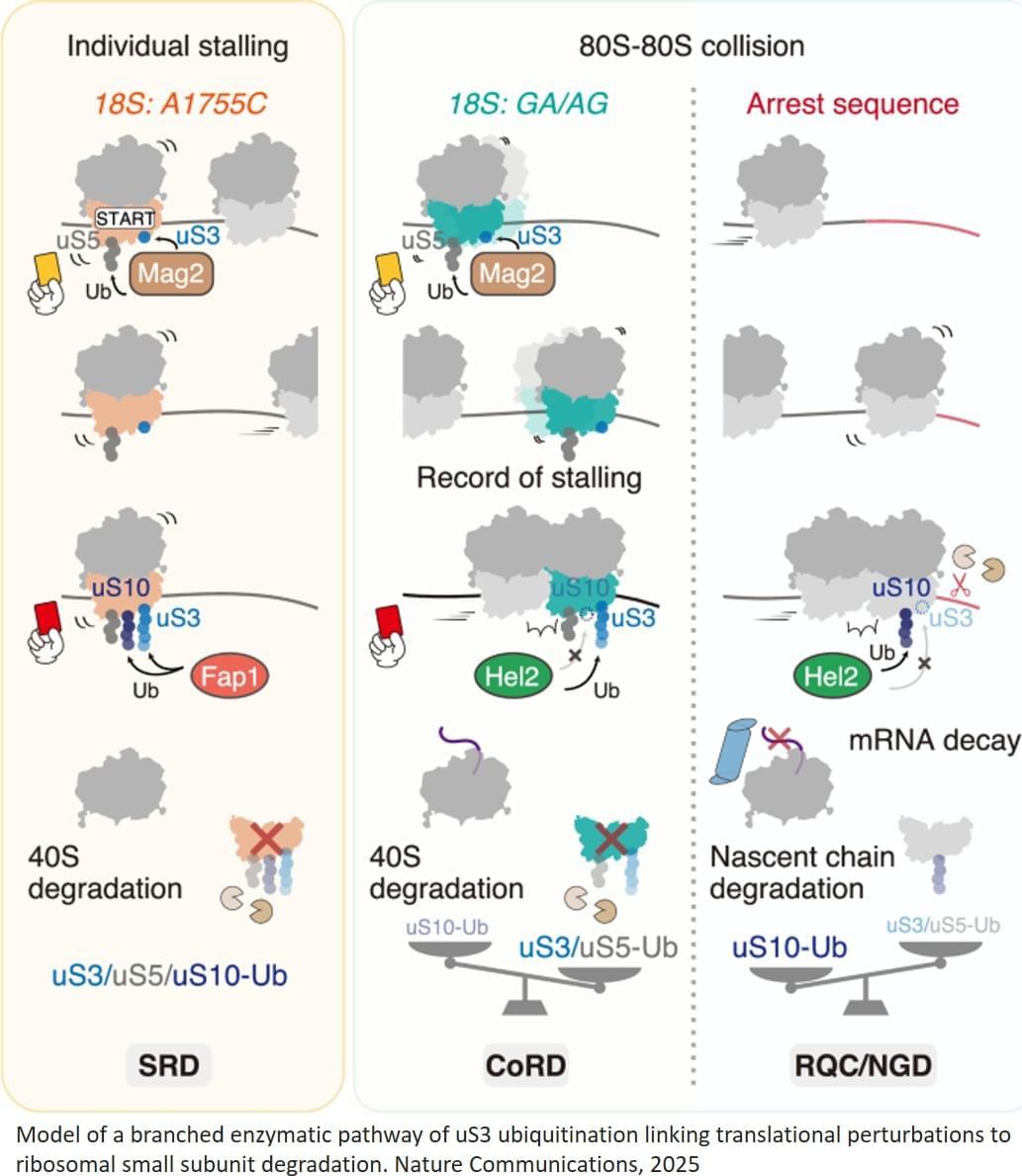
Breakthrough in DNA sequencing offers clues to why most smokers do not develop lung cancer.
“Our data suggest that these individuals may have survived for so long in spite of their heavy smoking because they managed to suppress further mutation accumulation,” says pulmonologist and genetics researcher Simon Spivack, a co-author on the study. “This leveling off of mutations could stem from these people having very proficient systems for repairing DNA damage or detoxifying cigarette smoke.”
Researchers who study the health effects of cigarette smoke have used all kinds of methods — from giving lab animals high doses of chemicals found in tobacco to combing through archives to determine which diseases smokers get more often — to figure out how the habit affects the body. Those studies have made it clear that cigarettes contain hundreds of harmful chemicals, including dozens of carcinogens.
For decades, researchers didn’t have any way to measure the mutations in lung cells that actually cause lung cancer. Five years ago, researchers at Albert Einstein College of Medicine in New York found a way to overcome technical limitations that had made it impossible to sequence the genome. That is, they figured out how to determine the exact order of the A, T, C, and G molecules of the DNA within a single cell without introducing too many errors in the process.