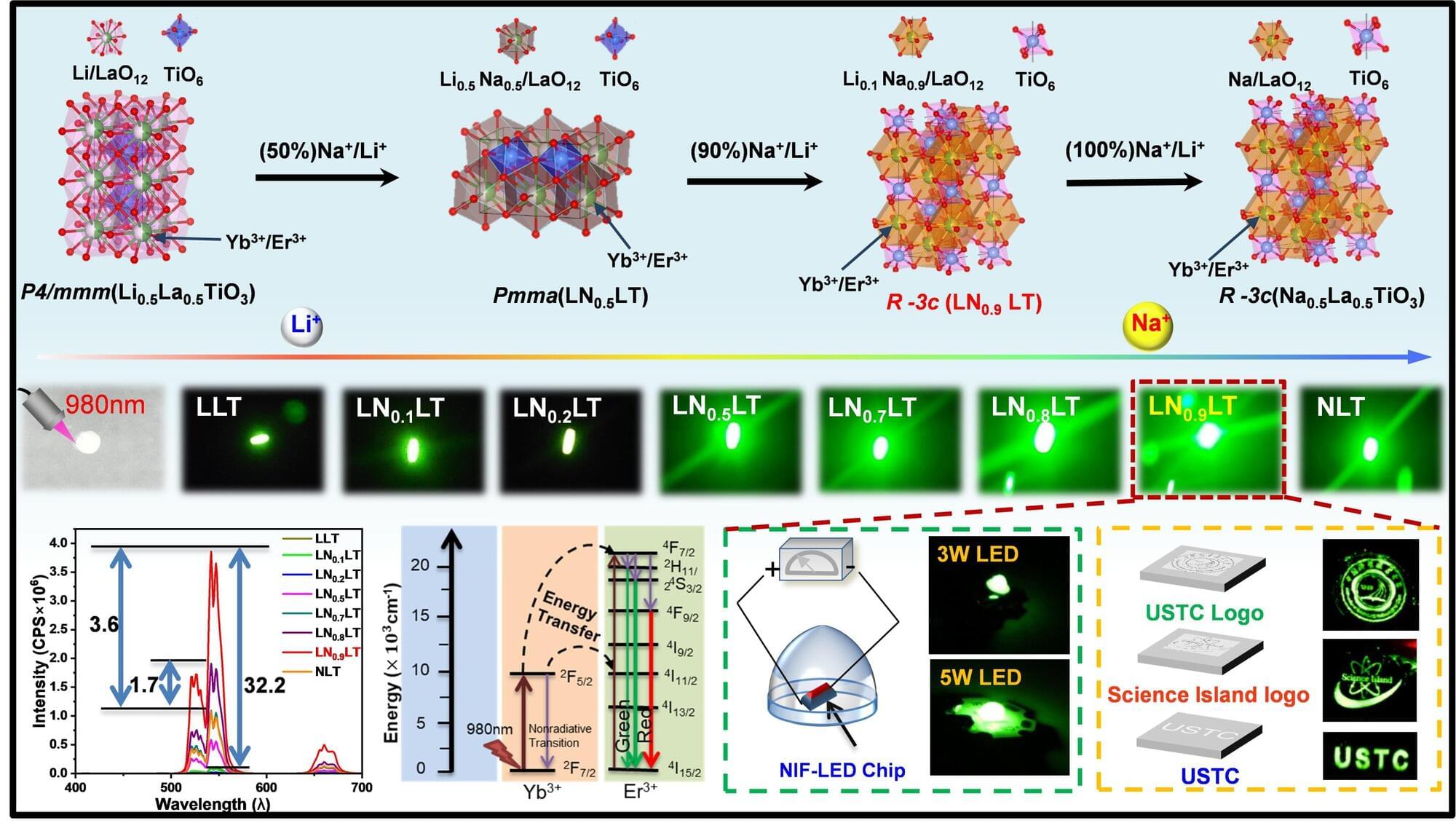
Researchers at the Hefei Institutes of Physical Science of the Chinese Academy of Sciences have developed a new way to significantly enhance upconversion luminescence in oxide perovskites, a class of materials known for their thermal and chemical stability but limited optical efficiency.
Led by Professor Jiang Changlong, the team introduced a dual-cation substitution strategy in titanate perovskites by precisely adjusting the sodium-to-lithium ratio at the crystal’s A-site. This controlled substitution triggers a structural transition that improves energy transfer between rare-earth ions, resulting in a marked increase in luminescence intensity and quantum yield.
The findings are published in Journal of Alloys and Compounds.