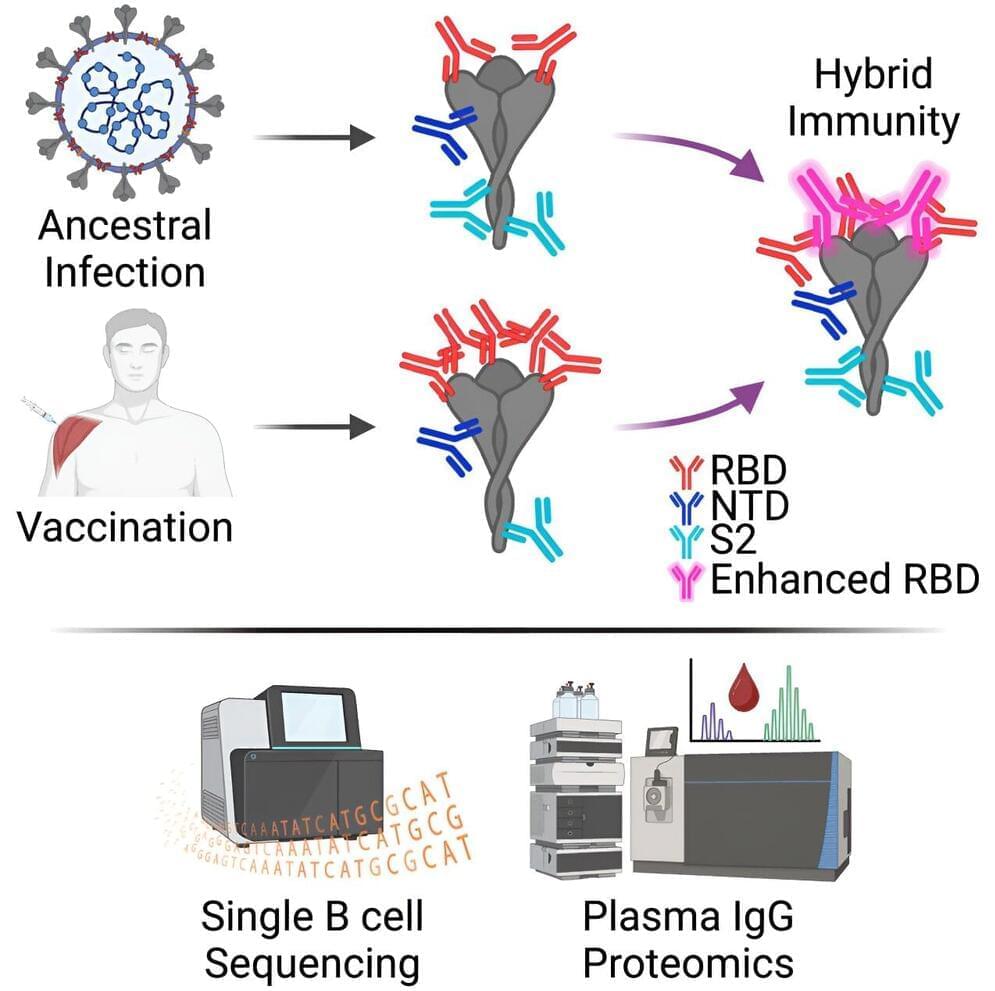
Researchers have discovered an antibody able to neutralize all known variants of SARS-CoV-2, the virus that causes COVID-19, as well as distantly related SARS-like coronaviruses that infect other animals.
As part of a new study on hybrid immunity to the virus, the large, multi-institution research team led by The University of Texas at Austin discovered and isolated a broadly neutralizing plasma antibody, called SC27, from a single patient. Using technology developed over several years of research into antibody response, the team led by UT engineers and scientists obtained the exact molecular sequence of the antibody, opening the possibility of manufacturing it on a larger scale for future treatments.
“The discovery of SC27, and other antibodies like it in the future, will help us better protect the population against current and future COVID variants,” said Jason Lavinder, a research assistant professor in the Cockrell School of Engineering’s McKetta Department of Chemical Engineering and one of the leaders of the new research, which was recently published in Cell Reports Medicine.