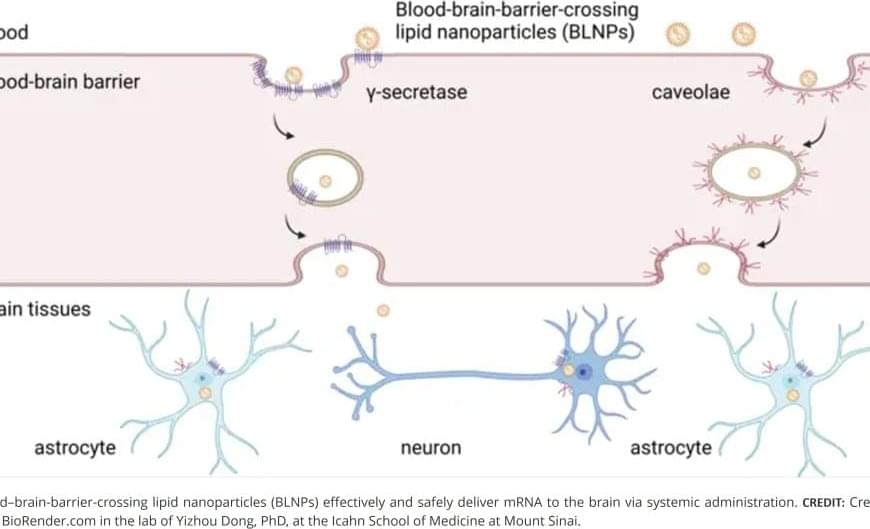
Getting mRNA into the brain could allow scientists to instruct brain cells to produce therapeutic proteins that can help treat or prevent disease by replacing missing proteins, reducing harmful ones, or activating the body’s defenses.
The research team designed and tested a library of lipids to optimize their ability to cross the blood-brain barrier. Through a series of structural and functional analyses, they identified a lead formulation, termed MK16 BLNP, that exhibited significantly higher mRNA delivery efficiency than existing lipid nanoparticles approved by the Food and Drug Administration (FDA). This system takes advantage of natural transport mechanisms within the blood-brain barrier, including caveolae-and γ-secretase-mediated transcytosis, to move nanoparticles across the barrier, say the investigators.
In studies using mouse models of disease, the BLNP platform successfully delivered therapeutic mRNAs to the brain, demonstrating its potential for clinical application.
Scientists have developed a lipid nanoparticle system capable of delivering messenger RNA (mRNA) to the brain via intravenous injection, a challenge that has long been limited by the protective nature of the blood-brain barrier.
The findings, in mouse models and isolated human brain tissue, were published in Nature Materials. They demonstrate the potential of this technology to pave the way for future treatments for a wide range of conditions such as Alzheimer’s disease, amyotrophic lateral sclerosis, brain cancer, and drug addiction.
The blood-brain barrier serves as a protective shield, preventing many substances—including potentially beneficial therapies—from reaching the brain. While previous research introduced a platform for transporting large biomolecules such as proteins and oligonucleotides into the central nervous system, this new study focuses on a different approach: using specially designed lipid nanoparticles to transport mRNA across the barrier.