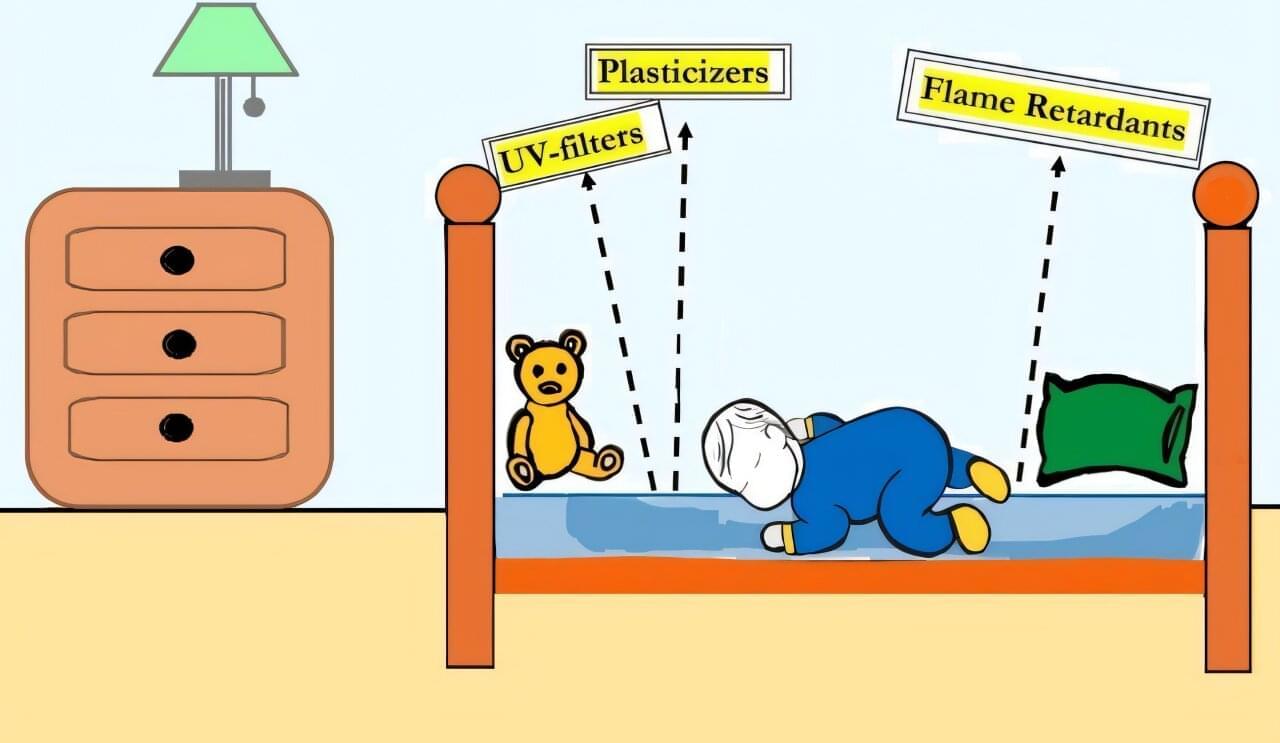
Babies and young children may breathe and absorb plasticizers called phthalates, flame retardants, and other harmful chemicals from their mattresses while they sleep, according to a pair of studies published by the University of Toronto in Environmental Science & Technology and Environmental Science & Technology Letters. These chemicals are linked to neurological and reproductive problems, asthma, hormone disruption, and cancer.
“Sleep is vital for brain development, particularly for infants and toddlers. However, our research suggests that many mattresses contain chemicals that can harm kids’ brains,” says senior author Miriam Diamond, professor at the University of Toronto.
“This is a wake-up call for manufacturers and policymakers to ensure our children’s beds are safe and support healthy brain development.”