A new study at Emory Vaccine Center gets into the bone marrow.
Category: biotech/medical – Page 319

Open House
Have you heard about the crazy guys who bought an entire tower to convert it into a vertical village? Yes, that’s us.
Do you want to walk the 16-floor tower and explore the space? Still on the fence, if you should become a citizen? Do you have questions about how you can get involved and co-create? Wanna hear updates on what happened in the last 2 weeks? This event is for you! 👩🚀
About us: We are transforming a 16-floor tower in the heart of San Francisco into a self-governed vertical village —a hub for frontier technologies and creative arts. 8 themed floors will be dedicated to creating tier-one labs, spanning AI, Ethereum, biotech, neuroscience, longevity, robotics, human flourishing, and arts & music. These floors will house innovators and creators pushing the boundaries of human potential in a post-AI-singularity world.
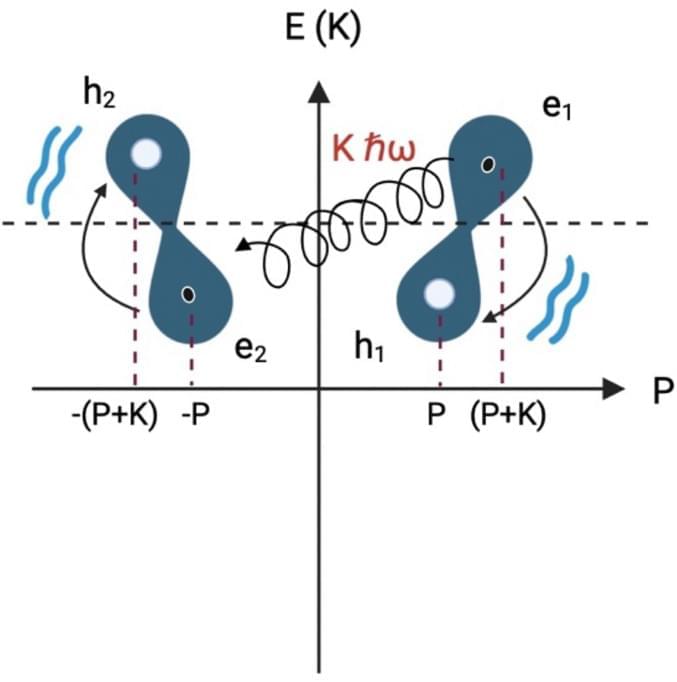
DNA as a perfect quantum computer based on the quantum physics principles
I believe that dna will be able to answer just about all our genetic coding questions so much that it will lead to even better breakthroughs in the future and use hardly any energy. I believe also that the master algorithm can eventually be derived from DNA as dna seems already a perfect master algorithm for human beings where human beings are the key to all future progress. I say this as quantum computing is still not stable but we already know that dna computers seem already a masterpiece already especially even organoids of the human brain. Really it becomes really quite simple as even the quantum realm is unstable but dna computers that are quantum would stabilize this currently unstable realm.
Riera Aroche, R., Ortiz García, Y.M., Martínez Arellano, M.A. et al. DNA as a perfect quantum computer based on the quantum physics principles. Sci Rep 14, 11,636 (2024). https://doi.org/10.1038/s41598-024-62539-5
Disease-Modifying, Neuroprotective Effect of N-Acetyl-l-Leucine in Adult and Pediatric Patients With Niemann-Pick Disease Type C
Background and ObjectivesN-acetyl-l-leucine (NALL) has been established to improve the neurologic manifestations of Niemann-Pick disease type C (NPC) after 12 weeks in a placebo-controlled trial. In the open-label extension phase (EP) follow-up, data were…
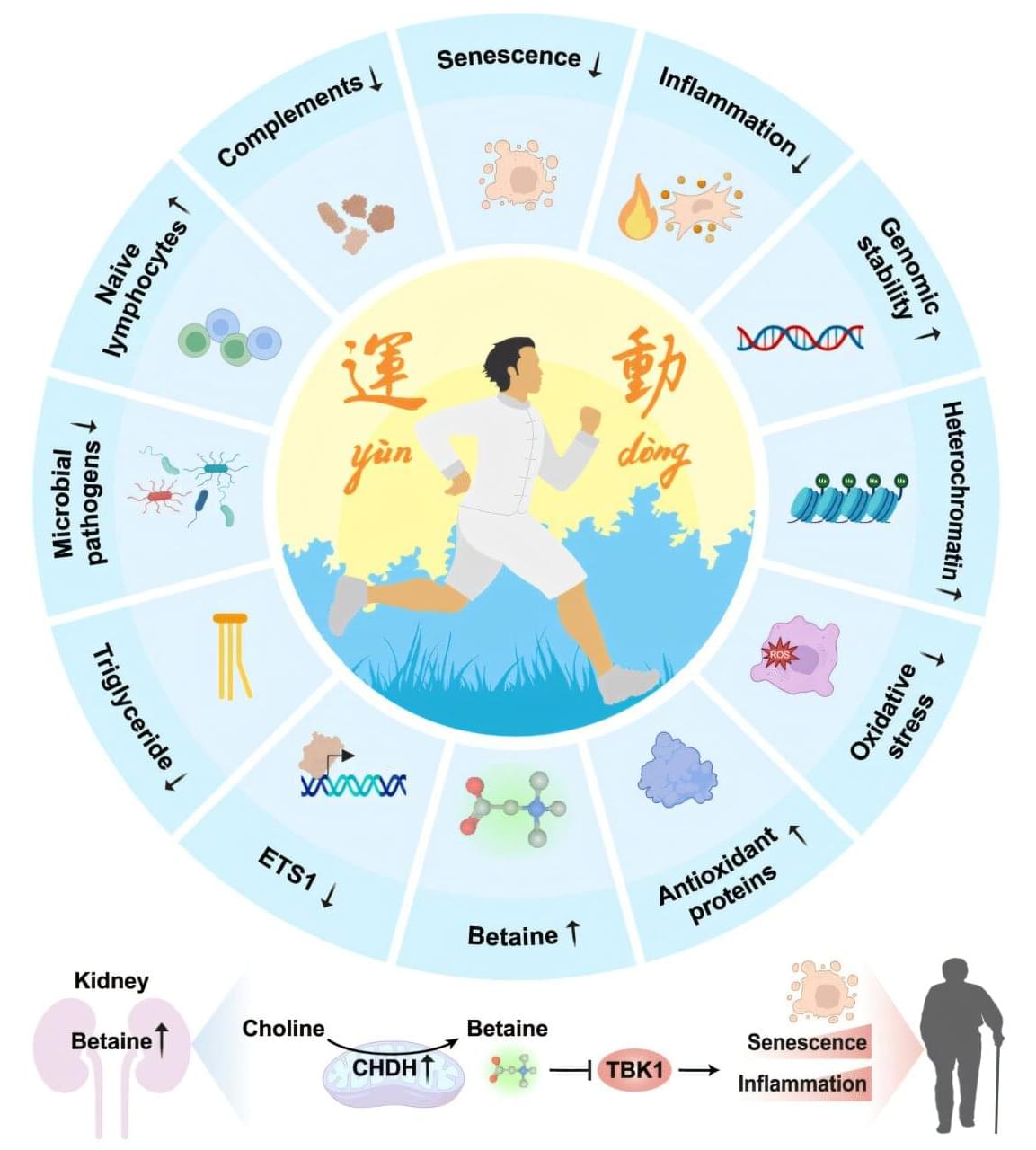
Mimicking the benefits of exercise with a single molecule
Capital Medical University, in collaboration with the Chinese Academy of Sciences, reports that betaine, a molecule produced in the kidney and enhanced through sustained exercise, operates as a potent inhibitor of inflammatory and aging-related pathways.
Regular physical activity boosts health across cardiovascular, metabolic, and neurological systems. Scientists have traced improvements in immune function, insulin sensitivity, clearing of senescent cells and tissue regeneration to consistent physical activity. Earlier animal studies suggested that long-term exercise can delay aging processes and reduce vulnerability to chronic disease.
Precise molecular explanations for how sustained exercise reshapes human biology remain incomplete. Many investigations have focused on single biomarkers or isolated tissues, leaving a need for systematic maps that can connect exercise to measurable physiological benefits. Specific factors capable of mimicking exercise’s protective effects without requiring continuous physical exertion have remained unclear.
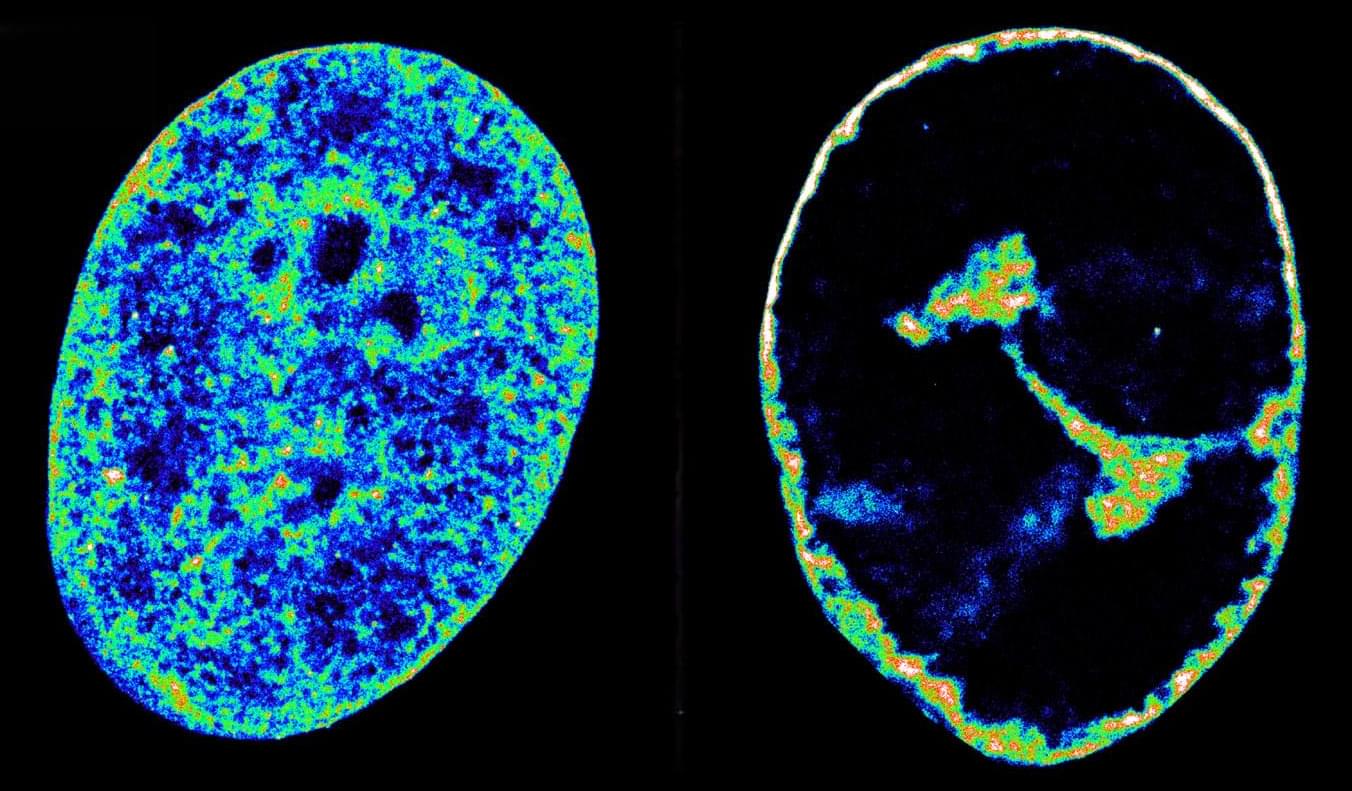

Blood stem cell mutations linked to lower risk of late-onset Alzheimer’s disease
A study published in Cell Stem Cell reveals that some mutations in blood stem cells might help protect against late-onset Alzheimer’s disease.
A team led by researchers at Baylor College of Medicine discovered that both a mouse model and people carrying blood stem cells with mutations in the gene TET2, but not in the gene DNMT3A, had a lower risk of developing Alzheimer’s disease. Their study proposes a mechanism that can protect against the disease and opens new avenues for potential strategies to control the emergence and progression of this devastating condition.
“Our lab has long been studying blood stem cells, also called hematopoietic stem cells,” said lead author Dr. Katherine King, professor of pediatrics— infectious diseases and a member of the Center for Cell and Gene Therapy and the Dan L Duncan Comprehensive Cancer Center at Baylor. She is also part of Texas Children’s Hospital.