Researchers have identified three anti-aging compounds produced by Paracoccus sanguinis, a bacterium found in the bloodstream.



Plant DNA has become a frontier for artificial intelligence, with large language models turning genetic sequences into interpretable content for researchers. These tools treat bases like words, revealing hidden patterns that once eluded traditional methods.
A study published by Dr. Meiling Zou from Hainan University describes how language-based models interpret extensive plant genomes with remarkable precision.
Not only can the drug metformin help to effectively manage type 2 diabetes, it may also give older women a better chance of living to the grand old age of 90, according to new research – thanks, it seems, to a variety of anti-aging effects.
The research used data from a long-term US study of postmenopausal women. Records on a total of 438 women were picked out – half who took metformin for their diabetes, and half who took a different diabetes drug, called sulfonylurea.
While there are a lot of caveats and asterisks to the study, those in the metformin group were calculated to have a 30 percent lower risk of dying before the age of 90 than those in the sulfonylurea group.
Envision this possible future clinical scenario: a breast cancer patient and her physicians are deciding on the best possible treatment. Their decision is informed by a comprehensive molecular profile of the patient’s cancer samples that predicts the most likely response of the cancer to treatment.
If the profile predicts a high likelihood of a complete positive response and long-term freedom from relapse, then this treatment would be the preferred choice. But if the profile predicts that the tumor would likely be resistant to treatment, alternative treatments must be implemented.
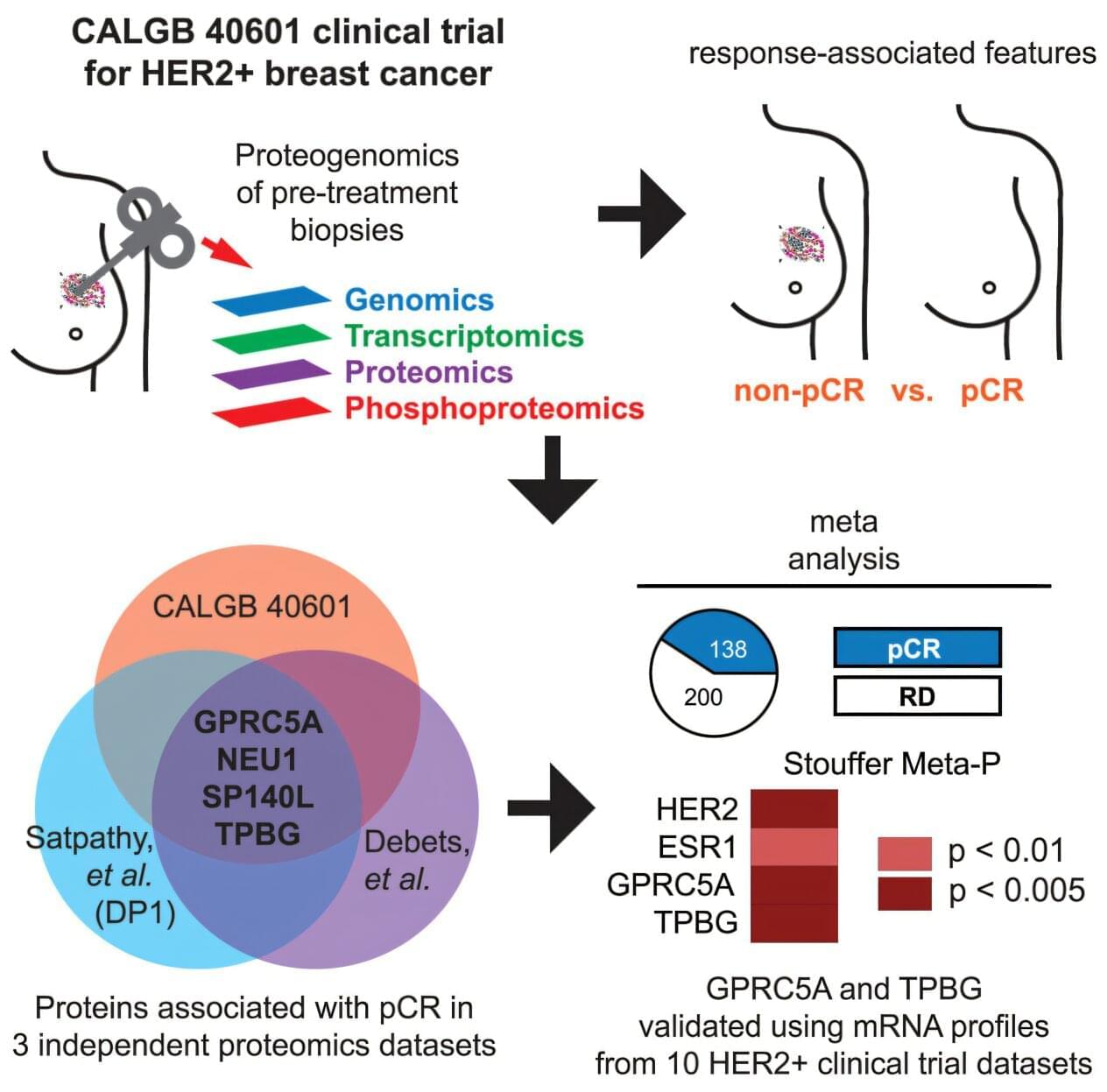
Although this situation is not yet a reality, a team led by researchers at Baylor College of Medicine and the Broad Institute of Massachusetts Institute of Technology and Harvard has taken significant steps in that direction. They report in Cell Reports Medicine that conducting an integrated proteogenomic profiling of cancer cells, which combines the analysis of DNA, RNA, protein and phosphoprotein data, revealed two novel indicators of tumor response to treatment and alternative therapeutic targets for treatment-resistant HER2+ breast cancer.

Critically ill patients with sepsis who are given statins may be more likely to survive, new research suggests.
Researchers set out to explore whether the cholesterol-busting drugs may bring additional benefits for patients.
The new study examined information on sepsis patients who received statins during a stint in intensive care and compared it with patients in a similar situation who did not receive statins.

As artificial intelligence (AI) tools shake up the scientific workflow, Sam Rodriques dreams of a more systemic transformation. His start-up company, FutureHouse in San Francisco, California, aims to build an ‘AI scientist’ that can command the entire research pipeline, from hypothesis generation to paper production.
Today, his team took a step in that direction, releasing what it calls the first true ‘reasoning model’ specifically designed for scientific tasks. The model, called ether0, is a large language model (LLM) that’s purpose-built for chemistry, which it learnt simply by taking a test of around 500,000 questions. Following instructions in plain English, ether0 can spit out formulae for drug-like molecules that satisfy a range of criteria.
Drug developed by Case Western Reserve University researchers found to protect ‘guardian of the brain’ Worldwide, more than 55 million people suffer from dementia caused by Alzheimer’s Disease (AD) and other conditions that destroy cells in the brain and nervous system. While there is no treatment to control or manage these neurodegenerative conditions, investigators at Case Western Reserve University, University Hospitals and the Louis Stokes Cleveland VA Medical Center have identified a new and promising drug to treat AD. The […]
A groundbreaking advancement in the field of vision restoration has recently emerged from the intersection of nanotechnology and biomedical engineering. Researchers have developed a novel retinal prosthesis constructed from tellurium nanowires, which has demonstrated remarkable efficacy in restoring vision to blind animal models. This innovative approach not only aims to restore basic visual function but also enhances the eye’s capability to detect near-infrared light, a development that holds promising implications for future ocular therapies.
The retina, a thin layer of tissue at the back of the eye, plays a crucial role in converting light into the electrical signals sent to the brain. In degenerative conditions affecting the retina, such as retinitis pigmentosa or age-related macular degeneration, this process is severely disrupted, ultimately leading to blindness. Traditional treatments have struggled with limitations such as electrical interference and insufficient long-term impacts. However, the introduction of a retinal prosthesis made from tellurium offers a fresh perspective on restoring vision.
Tellurium is a unique element known for its semiconductor properties, making it an excellent choice for developing nanostructured devices. The researchers carefully engineered tellurium nanowires and then integrated them into a three-dimensional lattice framework. This novel architecture facilitates easy implantation into the retina while enabling efficient conversion of both visible and near-infrared light into electrical impulses. By adopting this approach, the researchers ensured that the prosthesis would function effectively in various lighting conditions, a significant consideration for practical application in real-world scenarios.
“Scientists have shown that there is ultra-weak photon emission in the brain, but no one understands why the light is there.”
If light is at play and scientists can understand why, it could have major implications for medically treating brain diseases and drastically change the way physicians heal the brain. But measuring optical transport between neurons would be no easy task.
Our brain and nerves rely on incredibly fast electrical signals to communicate, a process long understood to involve tiny bursts of electricity called action potentials that travel along nerve fibers. But scientists are now exploring whether something else might also be part of this picture: light.
Yes—light, or more specifically, photons. Some researchers have suggested that nerves might not only use electrical impulses but could also send signals using photons, the same particles that make up visible light. This idea is based on the possibility that the fatty coating around nerves, called the myelin sheath, could act like an optical fiber—just like the cables used to carry internet signals using light.
In earlier work, the researchers behind this new study proposed that light might actually be generated in specific parts of the nerve called nodes of Ranvier, which are tiny gaps in the myelin sheath that help boost the electrical signal. Now, they’ve gone a step further: using a special photographic technique involving silver ions, they’ve found physical evidence of photons being emitted from these nodes during nerve activity.
Their experiments suggest that, alongside the familiar electrical signals, nerves might also be emitting light when they fire—shining a new light, literally and figuratively, on how our nervous system might work.
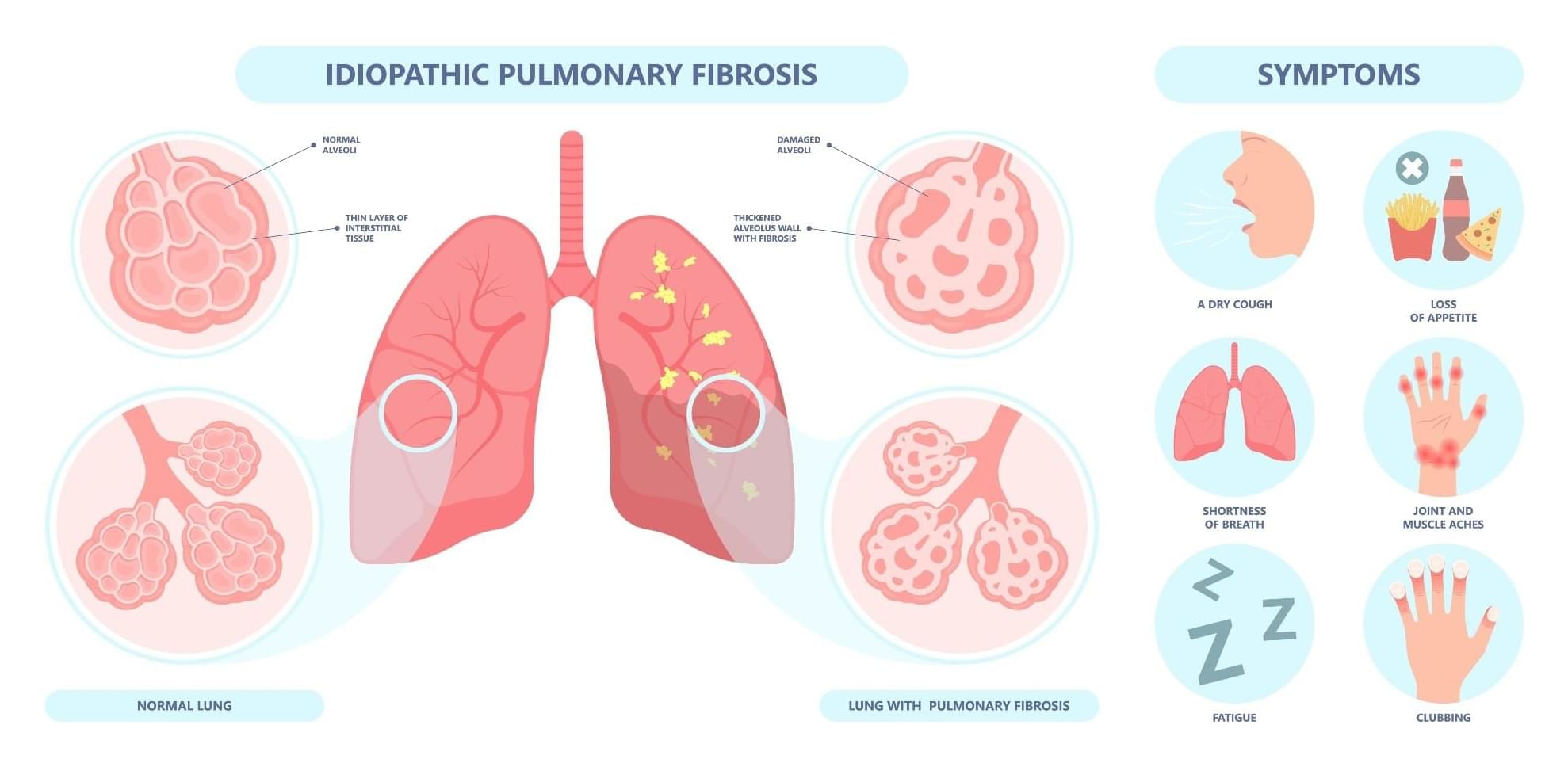
Researchers report that rentosertib, an AI-discovered TNIK inhibitor, showed promising safety and potential to improve lung function in patients with idiopathic pulmonary fibrosis in a 12-week phase 2a clinical trial. The highest dose group demonstrated a trend toward increased forced vital capacity, especially among patients not receiving standard antifibrotic therapy, supporting further clinical investigation.