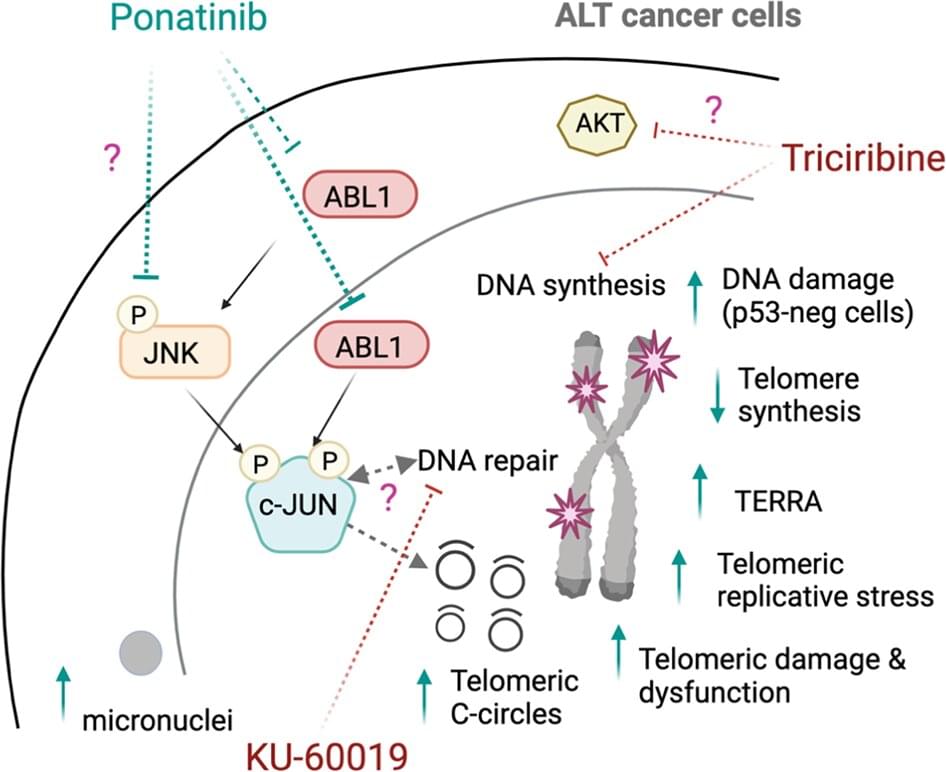
When asked how this model can cover such a broad scale, Xie says, that it “is rooted in the integration of mechanistic modeling and ML statistical methods, which allows the model to provide a more comprehensive and nuanced understanding of various aspects of RNA and related processes, while quantifying uncertainties due to limited knowledge.”
For example, she explains that, “The mechanistic aspect of the model captures intricate physical and chemical properties at the atomic level, which supports a deep understanding of the underlying biological processes, and the machine-learning element can effectively capture patterns in complex datasets—such as molecular simulations and single-molecule fluorescence microscopy time-course data—and learn relationships that might not be explicitly described in existing mechanistic models.”
In addition to helping scientists better understand the fundamental biology of RNA, the Northeastern team’s hybrid model promises many commercial benefits in the production of monoclonal antibodies, cell and gene therapies, and mRNA vaccines. As Xie says, “It can advance the knowledge of RNA manufacturing mechanisms and guide simultaneous design/control strategies at different levels, such as RNA sequence selection and specifications of critical quality attributes, with less experiments.”