Besides recognizing cancer signatures, liquid biopsy technologies are informing treatment decisions and predicting patient outcomes.


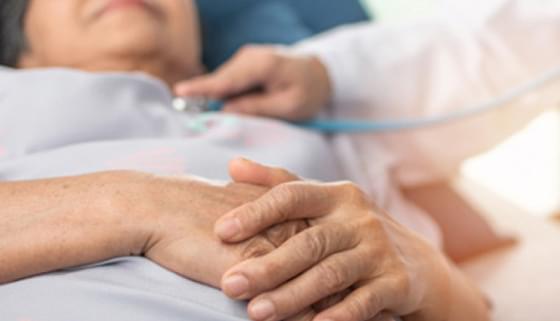
Congestive heart failure (also called heart failure) is a serious condition in which the heart doesn’t pump blood as efficiently as it should. Despite its name, heart failure doesn’t mean that the heart has literally failed or is about to stop working. Rather, it means that the heart muscle has become less able to contract over time or has a mechanical problem that limits its ability to fill with blood. As a result, it can’t keep up with the body’s demand, and blood returns to the heart faster than it can be pumped out—it becomes congested, or backed up. This pumping problem means that not enough oxygen-rich blood can get to the body’s other organs.
The body tries to compensate in different ways. The heart beats faster to take less time for refilling after it contracts—but over the long run, less blood circulates, and the extra effort can cause heart palpitations. The heart also enlarges a bit to make room for the blood. The lungs fill with fluid, causing shortness of breath. The kidneys, when they don’t receive enough blood, begin to retain water and sodium, which can lead to kidney failure. With or without treatment, heart failure is often and typically progressive, meaning it gradually gets worse.
More than 5 million people in the United States have congestive heart failure. It’s the most common diagnosis in hospitalized patients over age 65. One in nine deaths has heart failure as a contributing cause.
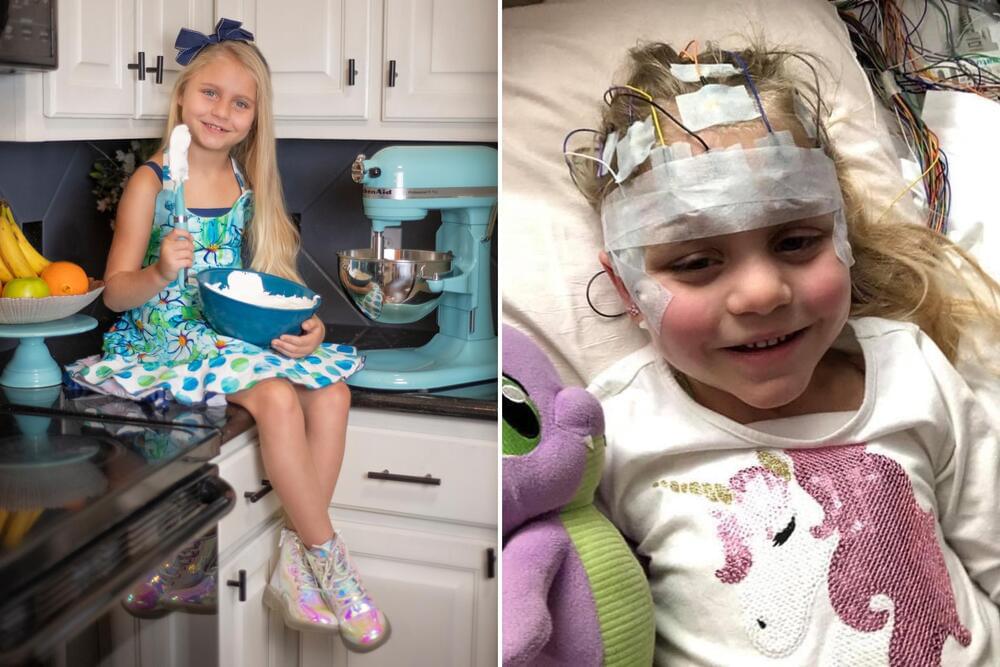
We don’t treat Isla’s vision loss as a sad circumstance or as something that is broken in her. It’s so important for us that she knows her vision impairment is not something that makes her less than. If anything, it makes her a stronger, more amazing person, and we couldn’t be prouder of who she is.
Vision impairment is the only symptom she displays of this disease, and we are fighting with everything we have to ensure it stays this way. We were told on diagnosis day that that day was the healthiest Isla would ever be, and that she was at her peak; two years later, and she has continued to defy that, Stockdale added.
The family believes that Isla’s incredible development is a result of the medication, called Miglustat, which she has been taking since November 2022.

In the study, published today in Science Translational Medicine, the researchers used engineered CAR T cells to target CD45—a surface marker found on nearly all blood cells, including nearly all blood cancer cells. Because CD45 is found on healthy blood cells too, the research team used CRISPR base-editing to develop a method called “epitope editing” to overcome the challenges of an anti-CD45 strategy, which would otherwise result in low blood counts, with potentially life-threating side effects. The early results represent a proof-of-concept for epitope editing, which involves changing a small piece of the target CD45 molecule just enough so that the CAR T cells don’t recognize it, but it… More.
A broad new strategy could hold hope for treating virtually all blood cancers with CAR T cell therapy, which is currently approved for five subtypes of blood cancer. A new preclinical, proof-of-concept study details the “epitope-editing” approach.
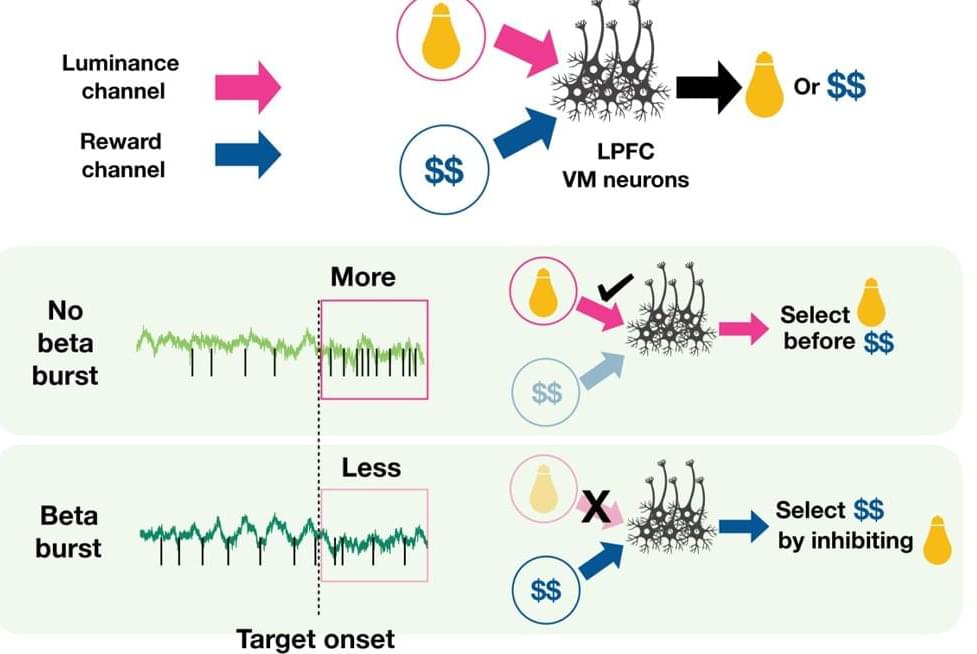
PHILADELPHIA—Trying to finish your homework while the big game is on TV? “Visual-movement” neurons in the front of your brain can help you stay focused, according to a new study from neuroscientists in the Perelman School of Medicine at the University of Pennsylvania.
In the study, published recently in Neuron, the scientists sought to illuminate the neural mechanism that helps the brain decide whether to focus visual attention on a rewarding task or an alluring distraction. By analyzing neuron activity in animal models as they faced this kind of attentional conflict, the researchers discovered that a pattern of coordinated activity called “beta bursts” in a set of neurons in the lateral prefrontal cortex (LPFC)—a section in the front of the brain responsible for motivation and rewards—appears to have a major role in keeping attention task-focused, essentially by suppressing the influence of the distracting stimulus.
“Our research suggests that while all brains have the ability to focus on a rewarding task and filter out distractions, some are better at it than others,” said senior author Bijan Pesaran PhD, the Robert A Groff II Professor of Neurosurgery at Penn Medicine. “By understanding how our brains process rewarding stimuli, we hope to be able to also understand failures to do so in a variety of cognitive and psychiatric disorders, including attention deficit disorder, schizophrenia, and obsessive-compulsive disorder.”

Excitingly, the researchers told New Scientist that if kept out of UV light, the products have the potential to last for a very long time. When it ultimately comes time to sunset the device, the substrate can simply be placed in soil, where it will biodegrade — thus naturally separating from the more recyclable computer components that the substrates hold.
The results have been promising. According to a press release, the material was tested by soldering a standard computer chip into it — and the researchers say the mushroom skin did pretty a solid job. And though it’s not ready for production just yet, the hope is that one day this mycelium material will become the substrate norm for printed circuit boards, flexible electronics, and even some medical devices.
“The prototypes produced are impressive,” Andrew Adamatzky, a computer scientist at the University of the West of England, told New Scientist, “and the results are groundbreaking.”
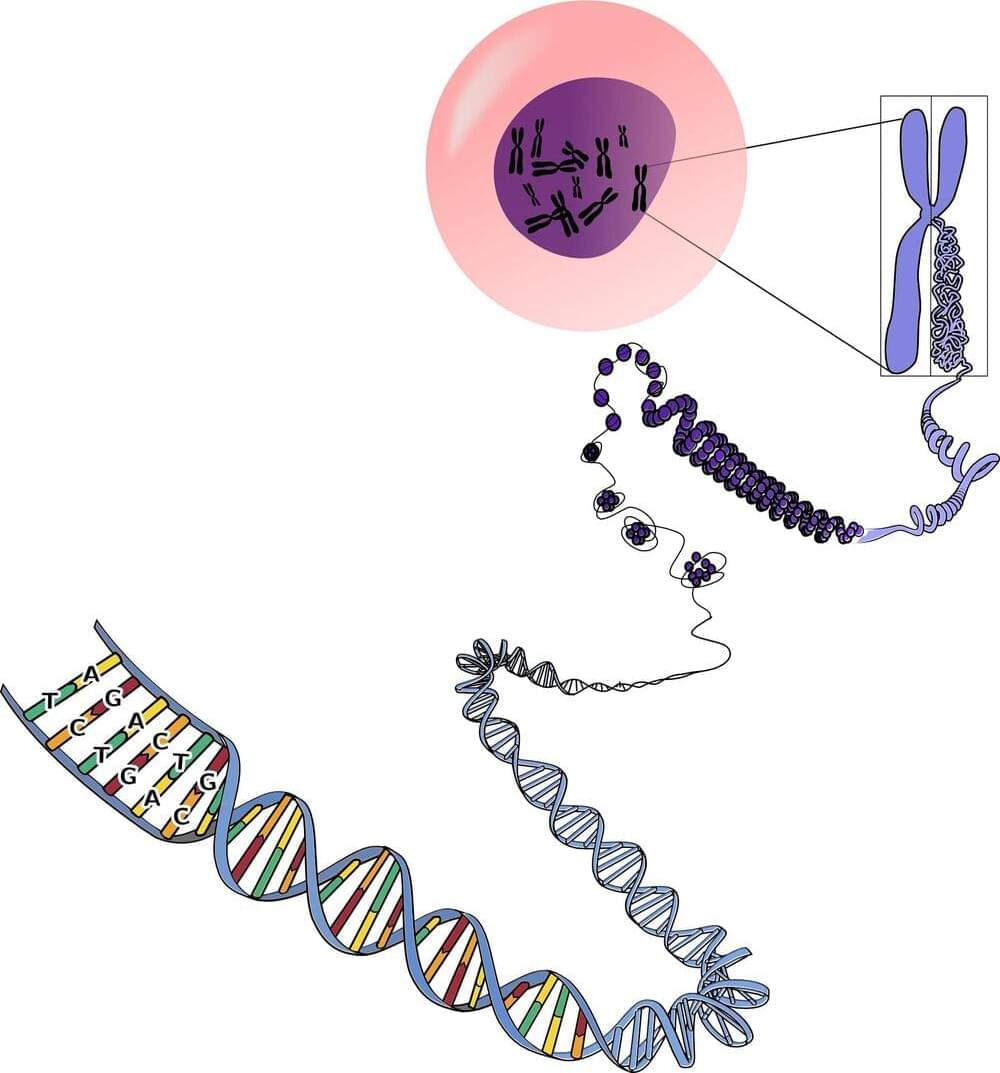
The Y chromosome is a never-ending source of fascination (particularly to men) because it bears genes that determine maleness and make sperm. It’s also small and seriously weird; it carries few genes and is full of junk DNA that makes it horrendous to sequence.
However, new “long-read” sequencing techniques have finally provided a reliable sequence from one end of the Y to the other. The paper describing this Herculean effort has been published in Nature.
The findings provide a solid base to explore how genes for sex and sperm work, how the Y chromosome evolved, and whether—as predicted—it will disappear in a few million years.
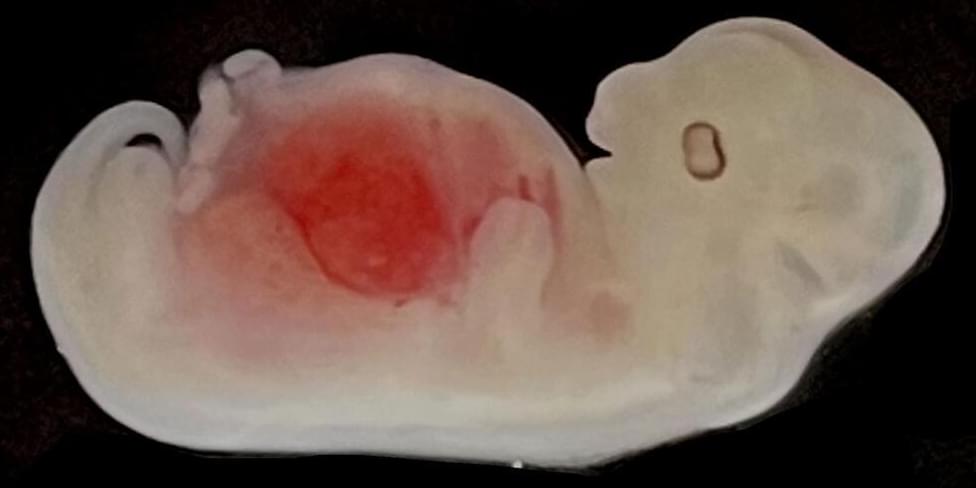
Scientists have successfully grown kidneys made of mostly human cells inside pig embryos — taking researchers yet another step down the long road toward generating viable human organs for transplant.
The results, reported September 7 in Cell Stem Cell, mark the first time a solid humanized organ, one with both human and animal cells, has been grown inside another species.
The work represents an important advance in the methods needed to grow humanized kidneys, hearts, and pancreases in animals.

Cells hidden in the skull may point to a way to detect, diagnose and treat inflamed brains.
A detailed look at the skull reveals that bone marrow cells there change and are recruited to the brain after injury, possibly traveling through tiny channels connecting the skull and the outer protective layer of the brain. Paired with the discovery that inflammation in the skull is disease-specific, these new findings collectively suggest the skull’s marrow could serve as a target to track and potentially treat neurological disorders involving brain inflammation, researchers report August 9 in Cell.
New observations of skull cell signals and skull tunnels suggest bone marrow there could be used to monitor neurological diseases.

Recent advancements in gene editing technologies may lead to a cure for hemoglobinopathies, including sickle cell disease and β-thalassemia.
A collaborative study between researchers from St Jude Children’s Research Hospital (TN, USA) and the Broad Institute of MIT and Harvard (MA, USA) has shown that adenosine base editing could be more effective than other gene editing approaches such as CRISPR/Cas9 for treating sickle cell disease and β-thalassemia. Comparing five different gene editing strategies utilizing either Cas9 nucleases or adenine base editors in hematopoietic and progenitor stem cells, the team found that base editing yielded more favorable results.
Sickle cell disease and β-thalassemia arise due to mutations in the β-globin subunit of hemoglobin, resulting in defective red blood cells. Previous studies have shown that restoring the function of γ-globin, a hemoglobin submit expressed during fetal development, could hold therapeutic advantages for patients with sickle cell disease and β-thalassemia. During fetal development, γ-globin combines with α-globin to form fetal hemoglobin. Following birth, expression of γ-globin ceases as it is replaced by β-globin to form adult hemoglobin. The researchers sought to see whether fetal hemoglobin expression could be restored in post-natal red blood cells to counter the effects of the disease, offering a potentially universal therapeutic approach for the disease.