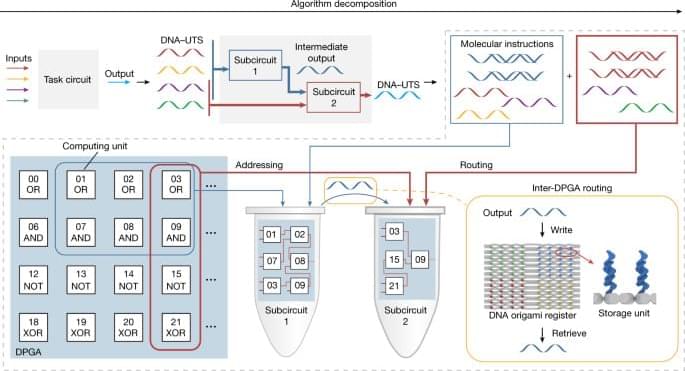
Generic single-stranded oligonucleotides used as a uniform transmission signal can reliably integrate large-scale DNA integrated circuits with minimal leakage and high fidelity for general-purpose computing.

Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links:
Oral Microbiome: https://www.bristlehealth.com/?ref=michaellustgarten.
Enter Code: ConquerAging.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
Use Code: ConquerAging At Checkout.
Epigenetic Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
At-Home Blood Testing (SiPhox Health): https://getquantify.io/mlustgarten.

Similarly, individuals who were diagnosed with diabetes at the age of 40 years died ten years earlier, and those diagnosed at the age of 50 died six years earlier than their healthy counterparts.
A robust association was established between earlier age of diabetes diagnosis and deaths due to vascular and non-neoplastic conditions. Common vascular diseases include stroke and myocardial infarction, while non-neoplastic conditions include neurological, respiratory, and infectious diseases.
The association between life expectancy and diabetes was marginally greater in women than in men. Compared to older adults, higher hazard ratios for mortality were associated with earlier age of diabetes detection.

In a recent study published in JAMA, researchers investigated whether accelerometer-assessed sedentary behavior was associated with incident dementia.
The global population is engaging in more sedentary-type activities such as sitting while using the computer, watching television, and driving. Studies have reported associations between sedentary behavior and cardiometabolic diseases and related mortality; however, its relationship with new-onset dementia is not clear.
The innovation – which has undergone advanced pre-clinical trials – is effective against a broad range of drug-resistant bacterial cells, including ‘golden staph’, which are commonly referred to as superbugs.
Antibiotic resistance is a major global health threat, causing about 700,000 deaths annually, a figure which could rise to 10 million deaths a year by 2050 without the development of new antibacterial therapies.
The new study led by RMIT University and the University of South Australia (UniSA) tested black phosphorus-based nanotechnology as an advanced infection treatment and wound healing therapeutic.
Results published in Advanced Therapeutics show it effectively treated infections,… More.
Researchers have invented a nano-thin superbug-slaying material that could be integrated into wound dressings to prevent or heal bacterial infections.
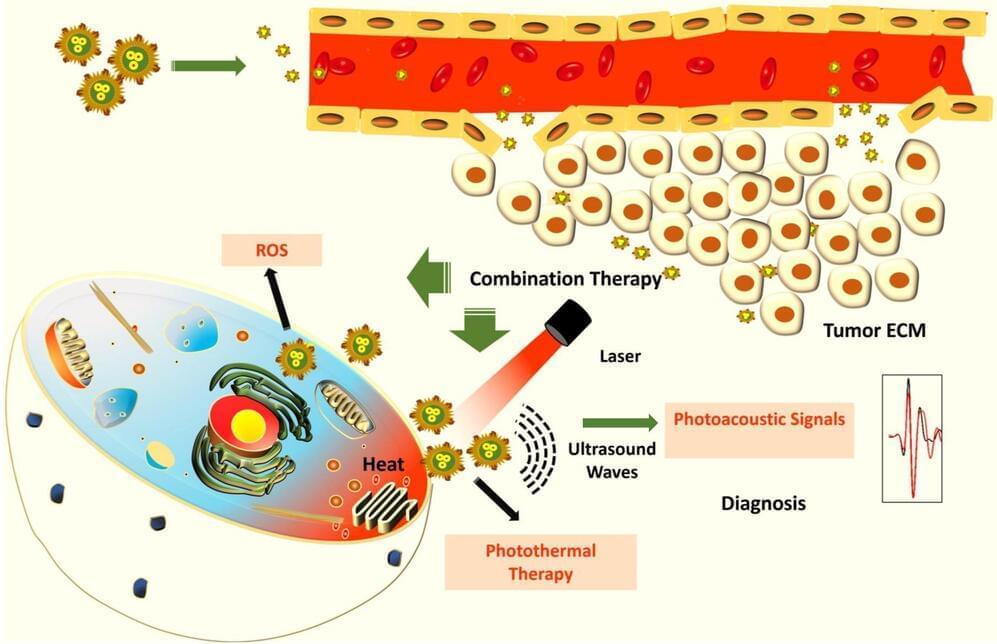
Scientists at the Indian Institute of Science (IISc) have developed a new approach to potentially detect and kill cancer cells, especially those that form a solid tumor mass. They have created hybrid nanoparticles made of gold and copper sulfide that can kill cancer cells using heat and enable their detection using sound waves, according to a study published in ACS Applied Nano Materials.
Early detection and treatment are key in the battle against cancer. Copper sulfide nanoparticles have previously received attention for their application in cancer diagnosis, while gold nanoparticles, which can be chemically modified to target cancer cells, have shown anticancer effects. In the current study, the IISc team decided to combine these two into hybrid nanoparticles.
“These particles have photothermal, oxidative stress, and photoacoustic properties,” says Jaya Prakash, Assistant Professor at the Department of Instrumentation and Applied Physics (IAP), IISc, and one of the corresponding authors of the paper. Ph.D. students Madhavi Tripathi and Swathi Padmanabhan are co-first authors.
In the 1939 neuroscientists began cutting living human brains in two in order to treat certain types of epileptic seizures. Subsequent experiments on those patients gave science an unnerving window into the nature of human consciousness. It turns out that there might be more versions inside of your own brain than you might be comfortable with.
#splitbrain #consciousness #malcovich #neuroscience.
https://landing.mailerlite.com/webforms/landing/h1g1p6
LINK LINK LINKS
Split brain behavioral experiments (Video of Joe)
Netherlands: The use of proton pump inhibitors (PPI) among kidney transplant recipients may lead to severe fatigue, fatigue severity, and lower mental and physical health-related quality of life, a new study has suggested. The study was published online in the American Journal Of Kidney Diseases.
Proton pump inhibitors (PPIs) are commonly prescribed medications for the management of acid-related gastrointestinal disorders.
Tim J. Knobbe and colleagues aimed to investigate the potential association between PPI use and fatigue as well as health-related quality of life among 937 kidney transplant recipients. Participants were at least one-year post-transplantation and were enrolled in the TransplantLines Biobank and Cohort Study.