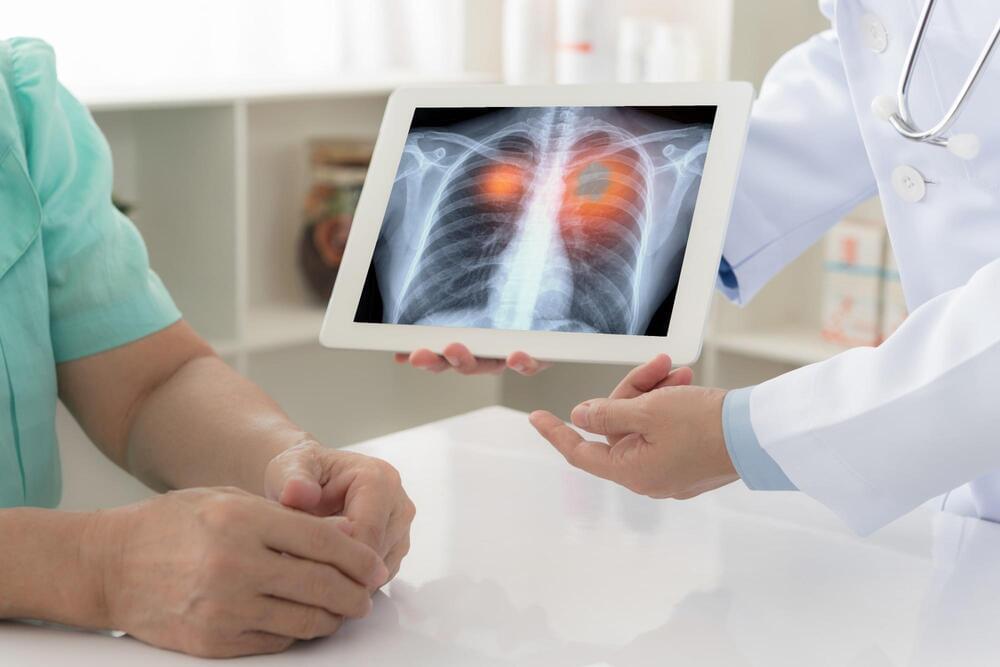
Adoptive cell therapy has emerged as a promising alternative treatment for hematological and solid cancers, with CAR-T therapy standing out as a prominent avenue. In this approach, T cells are genetically engineered with chimeric antigen receptors (CARs) to enhance their targeting capabilities1–2. The outcome of CAR-T cell therapy hinges on a complex interplay of phenotype, activation, and functional profiling of these engineered cells. Immunophenotypic characterization of CAR-T cells assumes a pivotal role in ensuring treatment quality and facilitating continuous monitoring of treatment response1. In the process of immunophenotyping, engineered T cells are separated based on their markers to characterize the composition of the cell population within the sample. The strategic identification and isolation of specific CAR-T cell subsets is essential in augmenting therapy responses2.
Deciphering Cellular Composition, Defining CAR-T Therapy Efficacy
Immunophenotyping is a pivotal technique that combines specific antibodies with fluorescent compounds to reveal specific protein expression in cell populations to identify categorize the tagged cells. Immunophenotyping leverages the differences in surface markers among T cells, reflecting their differentiation, activation, and memory status2. These markers provide insights into immune cell development, function, proliferation potential, and long-term viability. The distinct surface marker profiles closely correlate with the efficacy of CAR-T cell therapy3. Essential markers for immunophenotypic analysis, including CD3, CD4, CD8, CD45RA, CD34R0, CCR7, CD27, and CD95, are presented in Table 1.