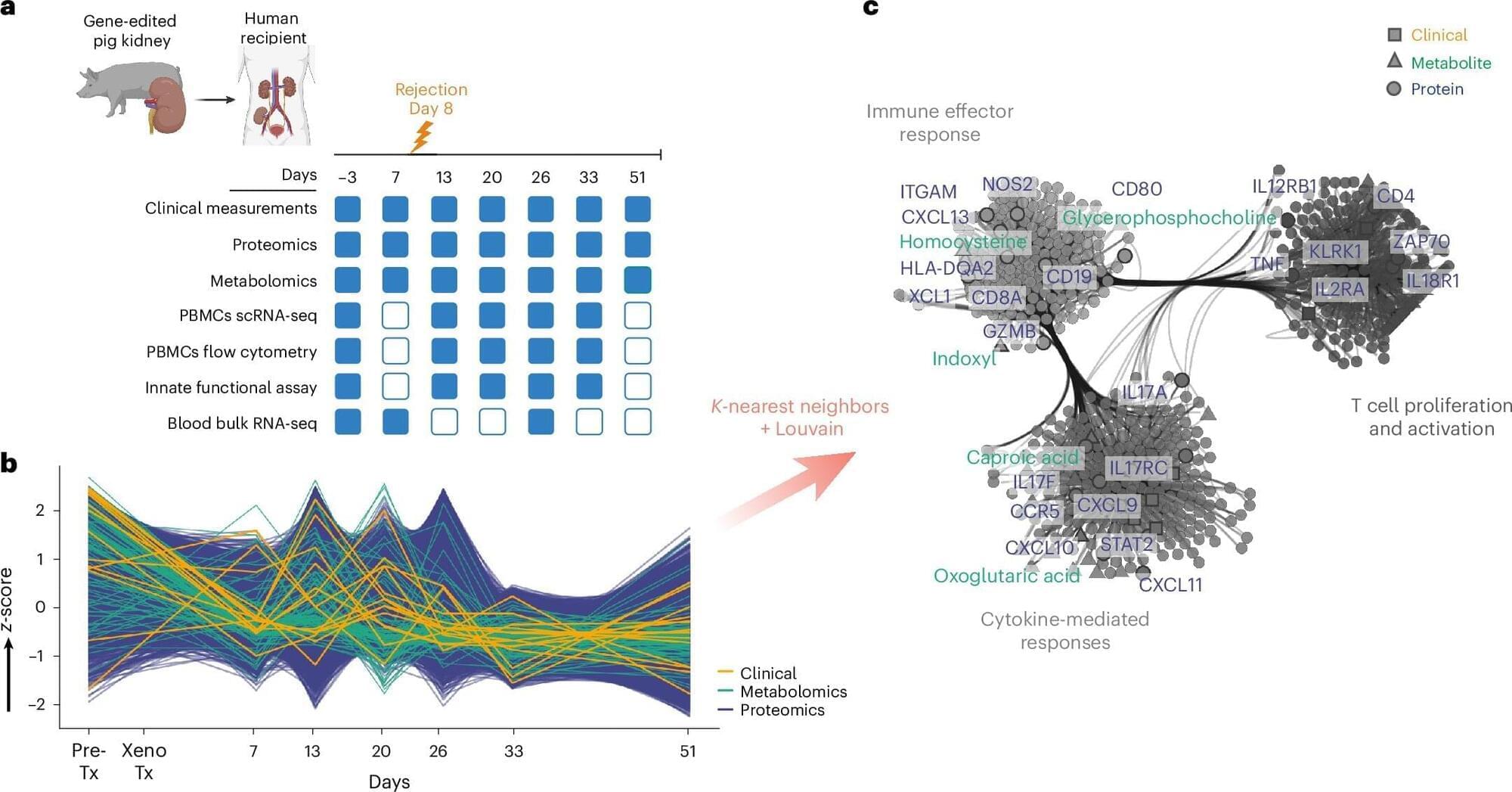
At SciCon 2024, John Cumbers, founder and CEO of SynBioBeta, explores the groundbreaking and controversial potential of synthetic biology and AI in brain and body replacement. He delves into stem cell research and AI’s role in regenerating brain function, while also addressing the provocative idea of gradually replacing parts of the brain and body. Cumbers discusses how these advancements could one day lead to life extension, challenging traditional views on aging, and raising ethical questions about the future of human biology.
SciCon (2024) is ResearchHub’s annual conference, which unites truth-seekers and innovators to push the boundaries of open science.
– ResearchHub’s mission is to accelerate the pace of scientific research. We are building a modern platform where people can collaborate on scientific research more efficiently, much like GitHub has done for software engineering. We believe scientific research should be accessible to everyone, collaborative, and prioritized.
Product: https://www.researchhub.com/
Website: https://researchhub.foundation/
GitHub: https://github.com/ResearchHub