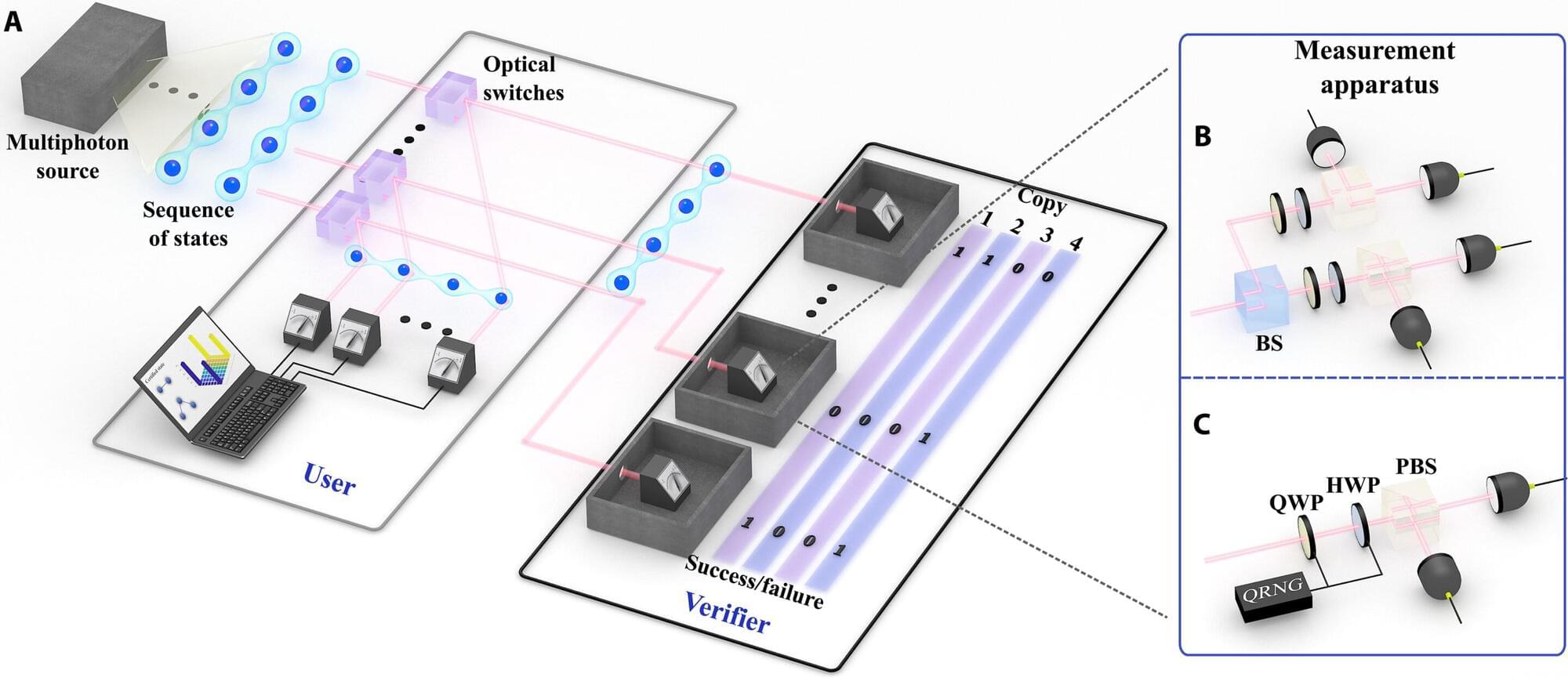
Many technological applications, such as sensors and batteries, greatly rely on electrochemical reactions. Improving these technologies depends on understanding how electrochemical reactions work. However, most current methods cannot look at electrochemical reactions in detail.
Scientists at Utrecht University have now developed a new method that overcomes this limitation. This provides a powerful new way to study and improve electrochemical processes. The study is published in PNAS.
Hydrogen production by water electrolysis is one example where electrochemical reactions at electrodes matter for sustainable technology. But the decisive steps happen within just a few nanometers of the electrode surface, which is too small for most conventional methods to resolve.