The finding could have implications on drug development beyond neuroscience.
A new study conducted by researchers at the University of Copenhagen has found that V-ATPase, an enzyme thought to be a key component of brain function, switches off randomly, even for hours at a time. This discovery has the potential to change our understanding of how our brain functions, according to a press release.
V-ATPase is an enzyme that can break down ATP molecules, the cell’s energy currency, as they pump protons across cellular membranes.
Evgenil Kovalev/iStock.
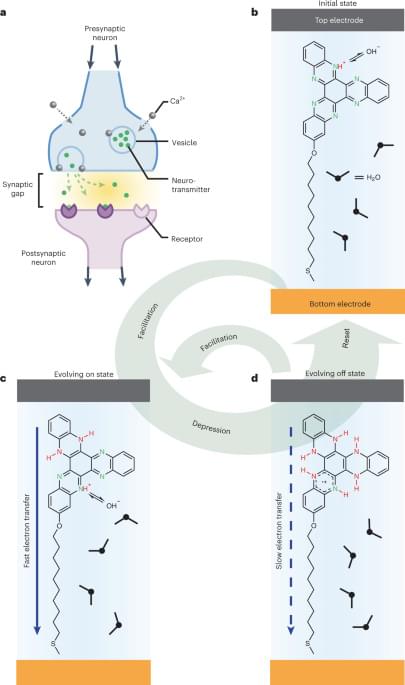
Simply put, the enzyme is responsible for providing energy to fill up the membrane bladders between neighboring neurons with chemicals that are needed to transfer a message between them. Therefore, the enzyme is quite crucial for neuronal communication, or that’s what researchers have thought so far.