Non-invasive methods, such as EEG and fNIRS, offer safer alternatives for BCI applications but must overcome obstacles related to signal quality and specificity.



The brain is regarded as one of the most complex known structures in the universe. It has billions of neurons, trillions of connections, and multiple levels ranging from cellular to molecular and synaptic. But the biggest challenge is that the brain is difficult to access.
“The brain is encased in a thick bone,” said Kinney, “and if you try to access, poke, or prod it, it will get really upset and hemorrhage, and delicate neurons will die.”
Nevertheless, Kinney said progress is being made on various fronts, particularly in the field of recording brain activity, which is good news for those trying to build brain-like computers.
A new study published in Psychological Science investigated the relationship between loneliness, brain activity, and social interactions. The results suggest that individuals who experience loneliness may process social information differently from those who do not, contributing to feelings of isolation and disconnection.
The study highlights the importance of social connection for psychological well-being. It emphasizes the need for further research in this area to develop effective interventions to help individuals experiencing loneliness improve their social connections and overall quality of life.
Humans are social creatures, and social connection is essential for physical and mental health. Social isolation and loneliness have been linked to various adverse outcomes, including depression, anxiety, cardiovascular disease, and even mortality.
A study in Australia found that men with anxiety disorders tended to have reduced bone mineral density in their lumbar spine and femoral neck bones. This association was found even when controlling for sociodemographic, biometric and lifestyle factors, other diseases, and medication use, but disappeared when participants with a history of mood disorders were excluded from the sample. The study was published in Acta Psychiatrica Scandinavica.
Bone mineral density refers to the quantity of minerals, primarily calcium and phosphorus, present in a segment of bone. It serves as an indicator of bone strength and density.
Studies have shown that certain psychiatric disorders might negatively impact bone health. These include unipolar depression, bipolar disorder, schizophrenia and anorexia nervosa. A meta-analytic review of 21 studies conducted in 2016 reported a very clear link between depression and reduced bone mineral density in several regions.
Scientists from a collection of Chinese research institutions collaborated on a study of organ regeneration in mammals, finding deer antler blastema progenitor cells are a possible source of conserved regeneration cells in higher vertebrates. Published in the journal Science, the researchers suggest the findings have applications in clinical bone repair. With the activation of key characteristic genes, it could potentially be used in regenerative medicine for skeletal, long bone or limb regeneration.
Limb and organ regeneration is a long coveted technology in medical science. Humans have some limited regenerative abilities, mostly in our livers. If a portion of the liver is removed, the remaining liver will begin to grow until it reaches its original functional size. Lungs, kidneys, and pancreas can do this also, though not as thoroughly or efficiently.
Compare this to a lizard regenerating a tail, a zebrafish replacing a fin, a lobster regrowing a claw, or an axolotl salamander that can rebuild organs, limbs, spinal cord and even missing brain tissue.
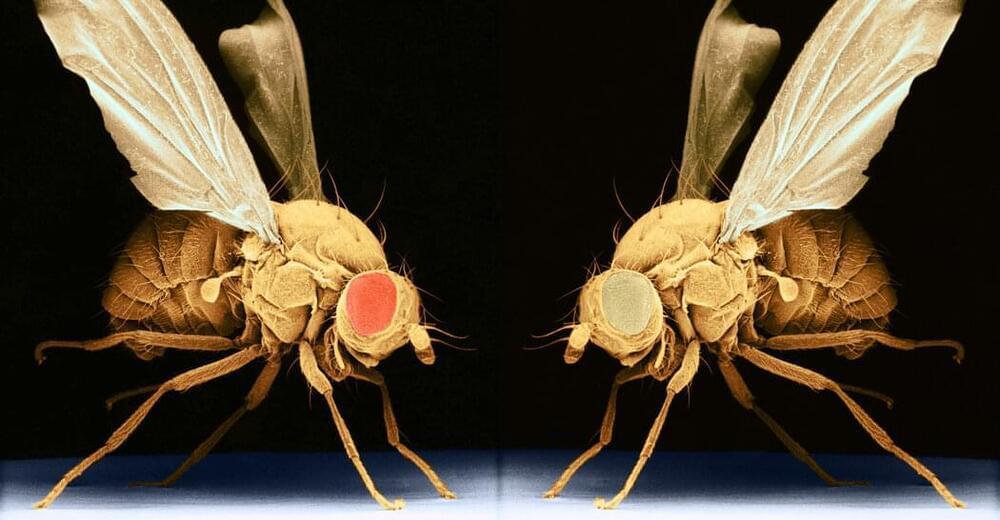

Psilocybin is a serotonergic psychedelic with untapped therapeutic potential. There are hints that the use of psychedelics can produce neural adaptations, although the extent and timescale of the impact in a mammalian brain are unknown. In this study, we used chronic two-photon microscopy to image longitudinally the apical dendritic spines of layer 5 pyramidal neurons in the mouse medial frontal cortex. We found that a single dose of psilocybin led to ∼10% increases in spine size and density, driven by an elevated spine formation rate. The structural remodeling occurred quickly within 24 h and was persistent 1 month later. Psilocybin also ameliorated stress-related behavioral deficit and elevated excitatory neurotransmission. Overall, the results demonstrate that psilocybin-evoked synaptic rewiring in the cortex is fast and enduring, potentially providing a structural trace for long-term integration of experiences and lasting beneficial actions.
Psilocybin is a classical psychedelic that shows promise as a treatment for depression. Shao et al. show that psilocybin administration leads to long-lasting modifications to the neural architecture in mice. The increases in the density and strength of neuronal connections may underlie the enduring behavioral effects of the compound.
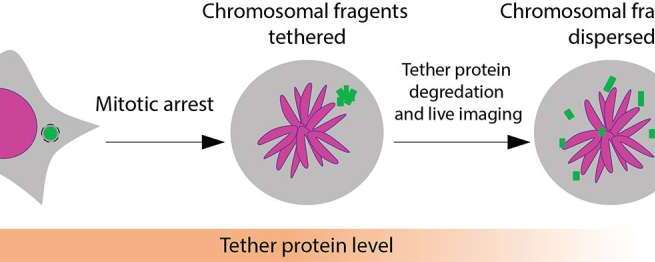
Healthy cells work hard to maintain the integrity of our DNA, but occasionally, a chromosome can get separated from the others and break apart during cell division. The tiny fragments of DNA then get reassembled in random order in the new cell, sometimes producing cancerous gene mutations.
This chromosomal shattering and rearranging is called “chromothripsis” and occurs in the majority of human cancers, especially cancers of the bones, brain and fatty tissue. Chromothripsis was first described just over a decade ago, but scientists did not understand how the floating pieces of DNA were able to be put back together.
In a study published in Nature, researchers at University of California San Diego have answered this question, discovering that the shattered DNA fragments are actually tethered together. This allows them to travel as one during cell division and be re-encapsulated by one of the new daughter cells, where they are reassembled in a different order.