Modern life replaced spirituality with goal-setting — and it’s making us depressed. Here’s how to win back your happiness.



“The new research program, led by Associate Professor Adeel Razi, from the Turner Institute for Brain and Mental Health, in collaboration with Melbourne start-up Cortical Labs, involves growing around 800,000 brain cells living in a dish, which are then “taught” to perform goal-directed tasks. Last year the brain cells’ ability to perform a simple tennis-like computer game, Pong, received global attention for the team’s research.”
Monash University-led research into growing human brain cells onto silicon chips, with new continual learning capabilities to transform machine learning, has been awarded almost $600,000 AUD in the prestigious National Intelligence and Security Discovery Research Grants Program.
According to Associate Professor Razi, the research program’s work using lab-grown brain cells embedded onto silicon chips, “merges the fields of artificial intelligence and synthetic biology to create programmable biological computing platforms,” he said.

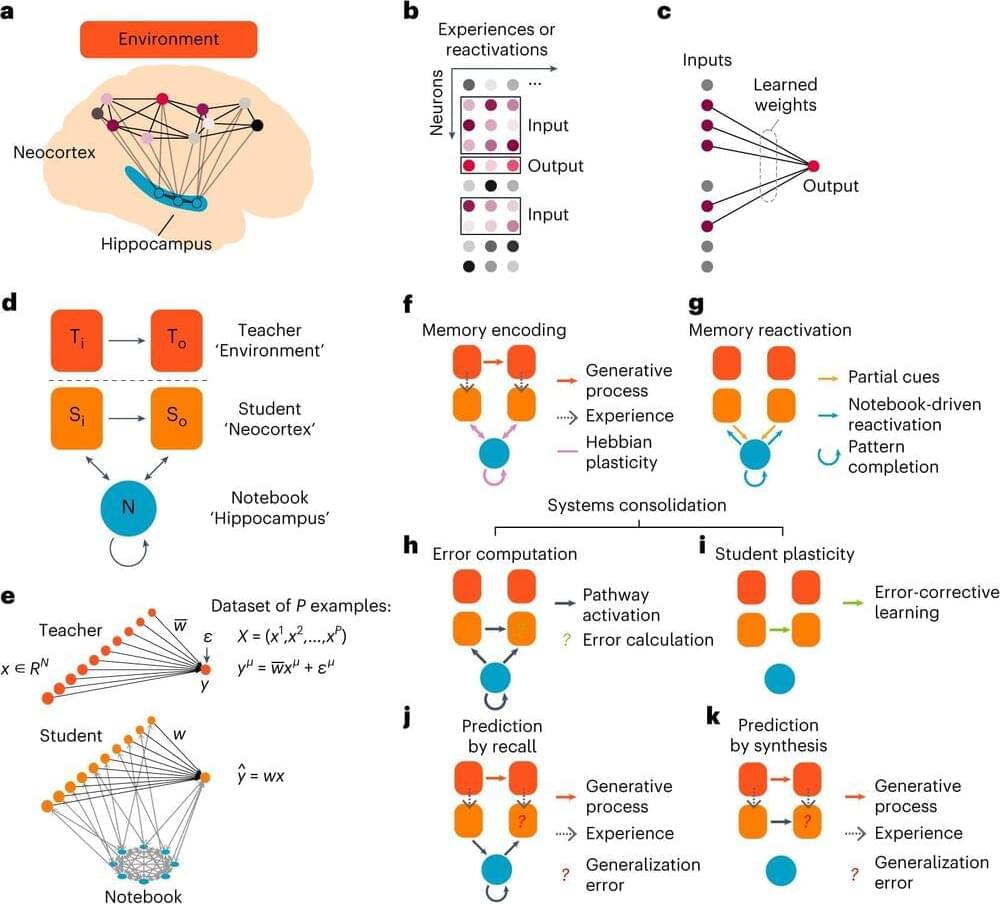
How useful a memory is for future situations determines where it resides in the brain, according to a new theory proposed by researchers at HHMI’s Janelia Research Campus and collaborators at UCL.
The theory, published in Nature Neuroscience, offers a new way of understanding systems consolidation, a process that transfers certain memories from the hippocampus —where they are initially stored—to the neocortex—where they reside long-term.
Under the classical view of systems consolidation, all memories move from the hippocampus to the neocortex over time. But this view doesn’t always hold up; research shows some memories permanently reside in the hippocampus and are never transferred to the neocortex.
Watch more interviews on the mystery of consciousness: https://t.ly/zGDTU
Consciousness is what we can know best and explain least. It is the inner subjective experience of what it feels like to see red or smell garlic or hear Beethoven. Consciousness has intrigued and baffled philosophers. To begin, we must define and describe consciousness. What to include in a complete definition and description of consciousness?
Free access to Closer To Truth’s library of 5,000 videos: https://closertotruth.com/
Support the show with Closer To Truth merchandise: https://bit.ly/3P2ogje.
Russ Hurlburt is a professor of psychology at the University of Nevada, Las Vegas. He received his PhD in clinical psychology from the University of South Dakota.
Register for free at CTT.com for subscriber-only exclusives: https://bit.ly/3He94Ns.
Abstract: I do not share the feeling that consciousness (whatever this means) cannot be understood in the context of the known physical laws. So far we do not understand it well, but neither do we fully understand thunderstorms, for that matter. I offer three small contributions in the direction of a direct naturalistic account of consciousness: (i) a purely physical account of agency and the openness of the future, which traces the source of information to past low entropy; (ii) a purely physical basis for a simple notion of “meaning”; and (iii) a suggestion that current understanding of quantum matter (without need of panpsychism) weakens the apparent hiatus between the mental and the physical.
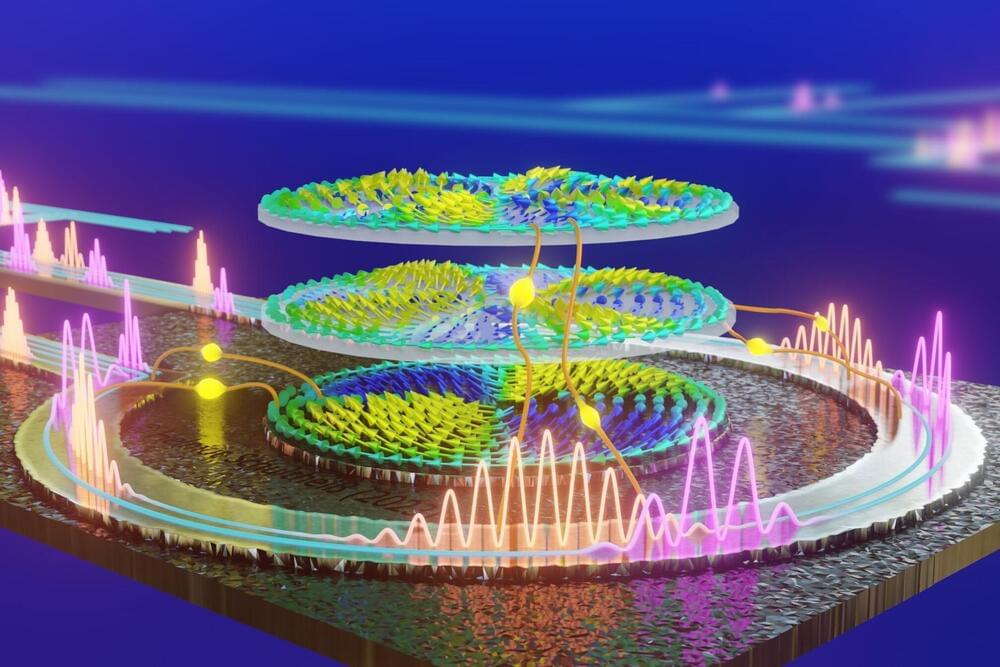
Neuromorphic computers do not calculate using zeros and ones. They instead use physical phenomena to detect patterns in large data streams at blazing fast speed and in an extremely energy-efficient manner.
In their project NIMFEIA, Katrin and Helmut Schultheiß along with their team from the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) have now taken this technology a tremendous step forward. They also demonstrated that their approach can be seamlessly integrated into conventional chip manufacturing. Their findings have now been published in Nature Communications.
What the researchers have developed at the HZDR-Institute of Ion Beam Physics and Materials Research is referred to by many names. “Neuromorphic computing,” for example, is one term, as the processes resemble those that occur within the brain. “Unconventional computing” is another name, as the technology is so different from the data processing that we are accustomed to today, which uses the Boolean logic of zeros and ones.
There’s a meditative state described in ancient Buddhist scriptures that is hard to imagine because it is not something – but nothing. Referred to as nirodha-samāpatti, it roughly translates as ‘the cessation of thought and feeling’, and it is the highest meditative state possible in Theravada Buddhism, following eight others called jhānas. Each jhāna requires deepening levels of concentration, and a retreat into the mind, away from typical consciousness.
According to David Vago, a psychologist at Vanderbilt University in Nashville and director of the Contemplative Neuroscience and Mind-Body Research Laboratory, nirodha-samāpatti refers to a ‘state of profound concentration or absorption in which all mental activity is temporarily suspended’. It’s said that the state leads to a total absence of sensation and awareness, which would help explain the stories of monks who stayed in this deep trance while fires burned around them.
There are many tales like these from religious texts. Vivid descriptions of dramatic alterations of consciousness that seem to defy our day-to-day experiences of mind and body. However, because the stories come from anecdotes, or ancient sources, it’s hard to know what’s true and what’s mythical. Recently however, psychologists and neuroscientists have begun to look for ways to find out what’s going on in the brain that might lead to such states.
For over a hundred years, scientists have held the belief that our thoughts, feelings, and dreams are shaped by the way various brain regions interact via a vast network of trillions of cellular connections.
However, a recent study led by the team at Monash University’s Turner Institute for Brain and Mental Health has examined more than 10,000 distinct maps of human brain activity and discovered that the overall shape of an individual’s brain has a much more substantial impact on our cognitive processes, emotions, and behavior than its intricate neuronal connectivity.
The study, recently published in the prestigious journal, Nature draws together approaches from physics, neuroscience, and psychology to overturn the century-old paradigm emphasizing the importance of complex brain connectivity, instead identifying a previously unappreciated relationship between brain shape and activity.