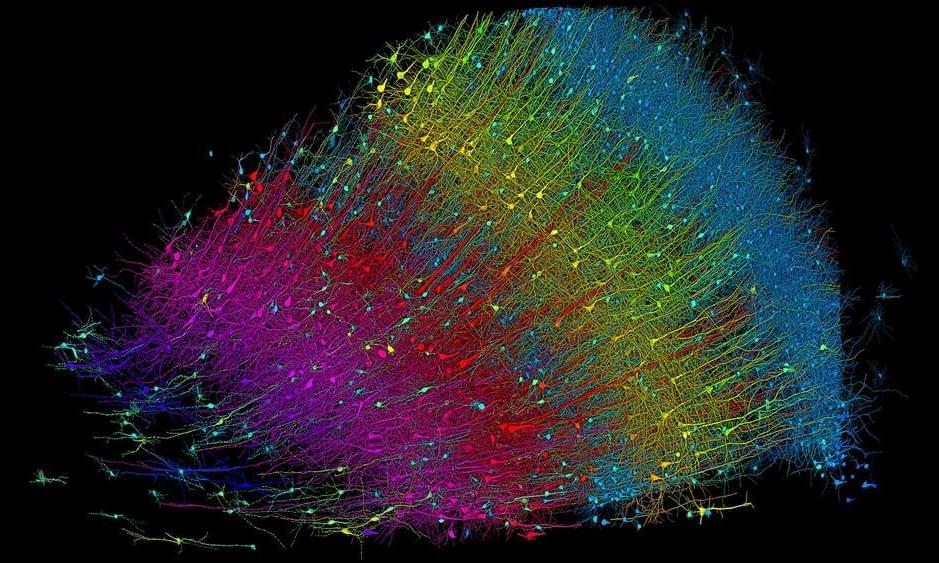
Explore the tiny ‘pizza slice’ down to the neuron.


Summary: Preschool children actively influence their own development to align with their genetic dispositions. By examining how toddlers interact with their environment, including activities like reading and puzzles, researchers found that children’s preferences impact how they engage in cognitive stimulation at home.
This active involvement helps shape their brain development alongside environmental factors. The findings emphasize the dynamic interplay between genetics and environment in early childhood, challenging the traditional views of passive developmental processes.

Neuralink’s first human patient has become so adept at using the company’s brain implant that he can now beat other players at video games.
On Wednesday, Elon Musk’s company provided a progress update on Noland Arbaugh, who received a brain implant in January that lets him remotely control the cursor on a laptop.
In March, Neuralink revealed that Arbaugh was using the implant to play games including Chess, Civilization VI, and Mario Kart. In Wednesday’s update, the company reported that Arbaugh’s use of the implant has only improved over time.
Does free will exist? Neil deGrasse Tyson and Chuck Nice sit down with astrophysicist Charles Liu sit down to discuss the existence of free will and whether physics allows for choice in our lives.
We explore cause and effect: how does uncertainty and chaos in the universe factor into free will? How important is the illusion of free will to society? What does a society that acknowledges a lack of free will look like?
Check out our second channel, @StarTalkPlus.
Get the NEW StarTalk book, ‘To Infinity and Beyond: A Journey of Cosmic Discovery’ on Amazon: https://amzn.to/3PL0NFn.
Support us on Patreon: / startalkradio.
FOLLOW or SUBSCRIBE to StarTalk:

A powerful new idea about how the laws of physics work could bring breakthroughs on everything from quantum gravity to consciousness, says researcher Chiara Marletto

To keep our bodies properly oriented, our brains perform impressive feats of calculation that track our stumbling meat sack through a mental map of our surrounds.
While a lot of research has focussed on the mapping, little has managed to determine how our neurological wiring monitors our direction within it.
A team of researchers from the University of Birmingham in the UK and the Ludwig Maximilian University of Munich in Germany has identified signature brain activity that describes a kind of ‘neural compass’ in the hope of understanding how we find our way through the world.
Researchers show that the possible cause of local bone erosion in cholesteatomas are fibroblasts from the bone that express a protein called activin A.
Chronic inflammation of the middle ear can cause several problems and complications that can affect a person’s hearing and balance. One such problem is the formation of a cholesteatoma, which is an abnormal collection of cells in the ear that can cause bone erosion if left untreated. In turn, this can cause symptoms such as hearing loss, dizziness, facial paralysis, and even a brain infection.
In a study published in the journal Nature Communications, researchers from Osaka University have revealed the cause of cholesteatomas, which may help in developing new therapies for patients who are suffering from this disease.

Reflections on a philosopher who believed we can solve the problem of consciousness.