Adult brains grow new neurons, and scientists have finally pinpointed where they come from


“This gives us evidence that the goal of vision is to establish a 3D understanding of an object,” said study senior author Ilker Yildirim, an assistant professor of psychology in Yale’s Faculty of Arts and Sciences.
“When you open your eyes, you see 3D scenes — the brain’s visual system is able to construct a 3D understanding from a stripped-down 2D view.”
Researchers have dubbed this process “inverse graphics,” describing how the brain’s visual processing system works like a computer graphics process, but in reverse, from a 2D image through a less view-dependent “2.5D” intermediate representation, and up to a much more view-tolerant 3D object.

Nerve cells are not just nerve cells. Depending on how finely we distinguish, there are several hundred to several thousand different types of nerve cell in the human brain according to the latest calculations. These cell types vary in their function, in the number and length of their cellular appendages, and in their interconnections. They emit different neurotransmitters into our synapses and, depending on the region of the brain – for example, the cerebral cortex or the midbrain – different cell types are active.
When scientists produced nerve cells from stem cells in Petri dishes for their experiments in the past, it was not possible to take their vast diversity into account. Until now, researchers had only developed procedures for growing a few dozen different types of nerve cell in vitro. They achieved this using genetic engineering or by adding signalling molecules to activate particular cellular signalling pathways. However, they never got close to achieving the diversity of hundreds or thousands of different nerve cell types that actually exists.
“Neurons derived from stem cells are frequently used to study diseases. But up to now, researchers have often ignored which precise types of neuron they are working with,” says Barbara Treutlein, Professor at the Department of Biosystems Science and Engineering at ETH Zurich in Basel. However, this is not the best approach to such work. “If we want to develop cell culture models for diseases and disorders such as Alzheimer’s, Parkinson’s and depression, we need to take the specific type of nerve cell involved into consideration.”
For the first time, researchers at ETH Zurich have successfully produced hundreds of different types of nerve cell from human stem cells in Petri dishes. In the future, it will thus be possible to investigate neurological disorders using cell cultures instead of animal testing.
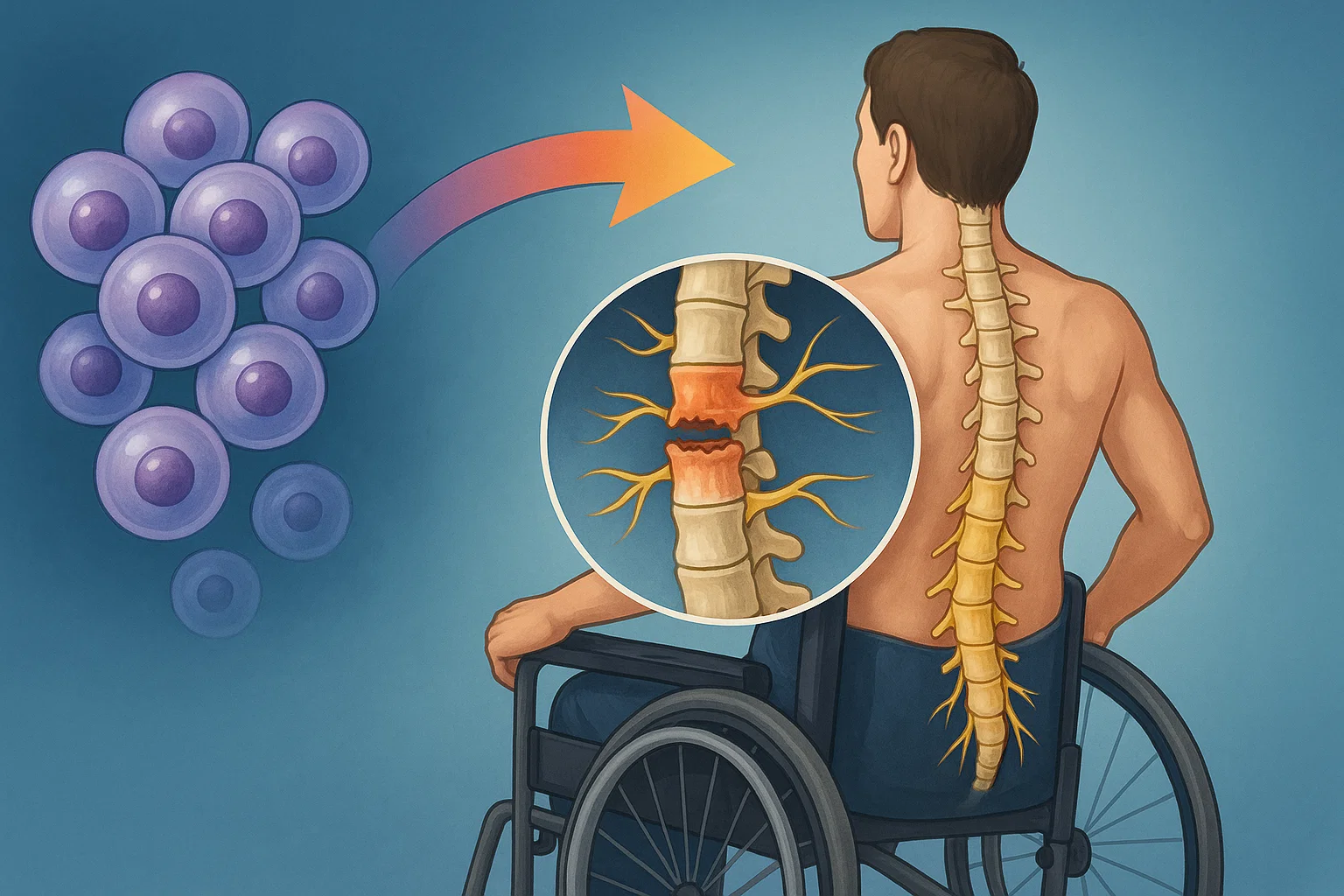
It begins with a fall, a crash, or a sudden jolt. In a split second, the spinal cord shatters. For millions, the damage is permanent. But in Shanghai and Suzhou, a group of scientists believes that might soon change.
This May, a biotech startup named XellSmart Biopharmaceutical received rare dual approval from both U.S. and Chinese regulators to launch a Phase I trial for an experimental treatment. The therapy is designed to repair spinal cord injuries using neurons grown in a lab.
The trial, described as the first of its kind, is being led by the Third Affiliated Hospital of Sun Yat-sen University in China. The goal: to test whether specialized nerve cells can be safely implanted into people whose spinal cords were recently injured.
A cell therapy for regenerating broken spinal cord using lab-grown neurons enters human trials for the first time.

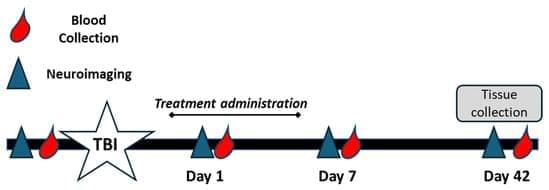
Background/Objectives: Traumatic brain injury (TBI) is a global leading cause of disability and death, with millions of new cases added each year. Oxidative stress significantly exacerbates primary TBI, leading to increased levels of intracerebral cell death, tissue loss, and long-term functional deficits in surviving patients. Catalase and superoxide dismutase (SOD) mitigate oxidative stress and play a critical role in dampening injury severity. This study examines the neuroprotective effects of the novel antioxidant alpha lipoic acid-based therapeutic, CMX-2043, on antioxidant enzymes in a preclinical TBI model via various drug administration routes. Methods: Piglets (n = 28) underwent cortical controlled impact to induce moderate–severe TBI and were assigned to placebo (n = 10), subcutaneous CMX-2043 (SQ, 10 mg/kg; n = 9), or intravenous CMX-2043 (IV, 9 mg/kg; n = 9) treatment groups. Treatments began 1 h after TBI induction and continued for 5 days. MRI was performed throughout the study period to evaluate brain recovery. Blood was collected at 1, 7, and 42 days post-TBI, and liver and brain tissues were collected at 42 days post-TBI to measure catalase and SOD activity. Results: CMX-2043 IV-treated piglets showed 46.3% higher hepatic catalase activity than placebo (p = 0.0038), while the SQ group did not show significant changes in hepatic catalase activity compared to placebo. In the brain, SQ-treated piglets had significantly higher catalase activity than both IV (p = 0.0163) and placebo (p = 0.0003) groups (45.8340 ± 3.0855, 36.4822 ± 1.5558, 31.6524 ± 1.3129 nmol/min/mg protein for SQ, IV, and placebo, respectively), while IV-treated piglets did not show significant changes compared to placebo. IV-treated piglets did exhibit 39.3% higher brain SOD activity than placebo (p = 0.0148), while the SQ group did not show a significant change. CMX-2043 treatment did not alter plasma antioxidant enzyme activity during the study period. Importantly, within CMX-2043 treated TBI groups, piglets with significantly decreased lesion volumes, midline shift, and combined swelling and atrophy had better brain recovery, determined by MRI on day 1, 7, and 42 days post-injury TBI, exhibited higher brain catalase activity at 42 days post-injury TBI regardless of administration route, suggesting a link between improved recovery and sustained local catalase activity. Conclusions: This study highlights the impact of administration route on tissue-specific antioxidant responses, with IV administration enhancing liver catalase and brain SOD activity, while SQ administration primarily elevated brain catalase activity. In addition, this study shows an association between increased brain catalase activity and decreased TBI brain lesioning, midline shift, and combined swelling and atrophy, thus emphasizing the role of antioxidant defenses in neuroprotection post-injury.