NIH-funded breakthrough could enable targeted therapies for many neurological disorders.


UNSW Sydney and Macquarie University psychology researchers have written an article warning that psychedelic therapies may switch on visual mental imagery in people with aphantasia and could raise the risk of intrusive thoughts, while calling for more detailed informed consent.
Known as a blind mind’s eye, people with aphantasia recall personal memories with fewer details and vividness. Visual mental imagery is absent. People with aphantasia cannot visualize objects, people, places, or memories, and they also recall personal memories with fewer details and vividness.
Recent reports, including one published case study and one pre-print along with anecdotal accounts, describe individuals with aphantasia gaining a new capacity to visualize after a single dose of ayahuasca or psilocybin, with positive self-reported outcomes during and after the experience, including within a year post-experience.

A large international study led by researchers at the Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, shows that major depressive disorder (MDD) not only increases risk for a wide range of diseases and social problems, but is also partly driven by factors such as loneliness, obesity, smoking, and chronic pain.
The study, published in Nature Mental Health, applied genetic methods to systematically test which traits are causes, and which are consequences, of depression. The findings highlight the double burden of MDD: it both arises from and contributes to poor health, making prevention and treatment particularly urgent.
“We show that depression sits at the center of a web of health problems,” says Joëlle Pasman, research associate at Amsterdam UMC and Karolinska Institutet, who led the study. “It is not only a debilitating condition in itself but also increases the risk of many diseases, while at the same time being triggered by social, behavioral, and medical factors.”

Memories of significant learning experiences—like the first time a driver gets a speeding ticket—are sharp, compared to the recollection of everyday events—like what someone ate for dinner two weeks ago. That’s because the human brain is primed to learn from helpful associations.
Carnegie Mellon University researchers have identified specific neural connections that are especially sensitive to this process of learning about causality. The discovery, while seemingly intuitive, could have widespread implications for understanding how humans learn and inform new ways to address learning challenges.
“If you look out the window and see dark clouds, you know that it’s going to rain and that you’ll need an umbrella,” said Eunsol Park, a Ph.D. student in the Department of Biological Sciences and the Center for the Neural Basis of Cognition, a joint program between Carnegie Mellon and the University of Pittsburgh.
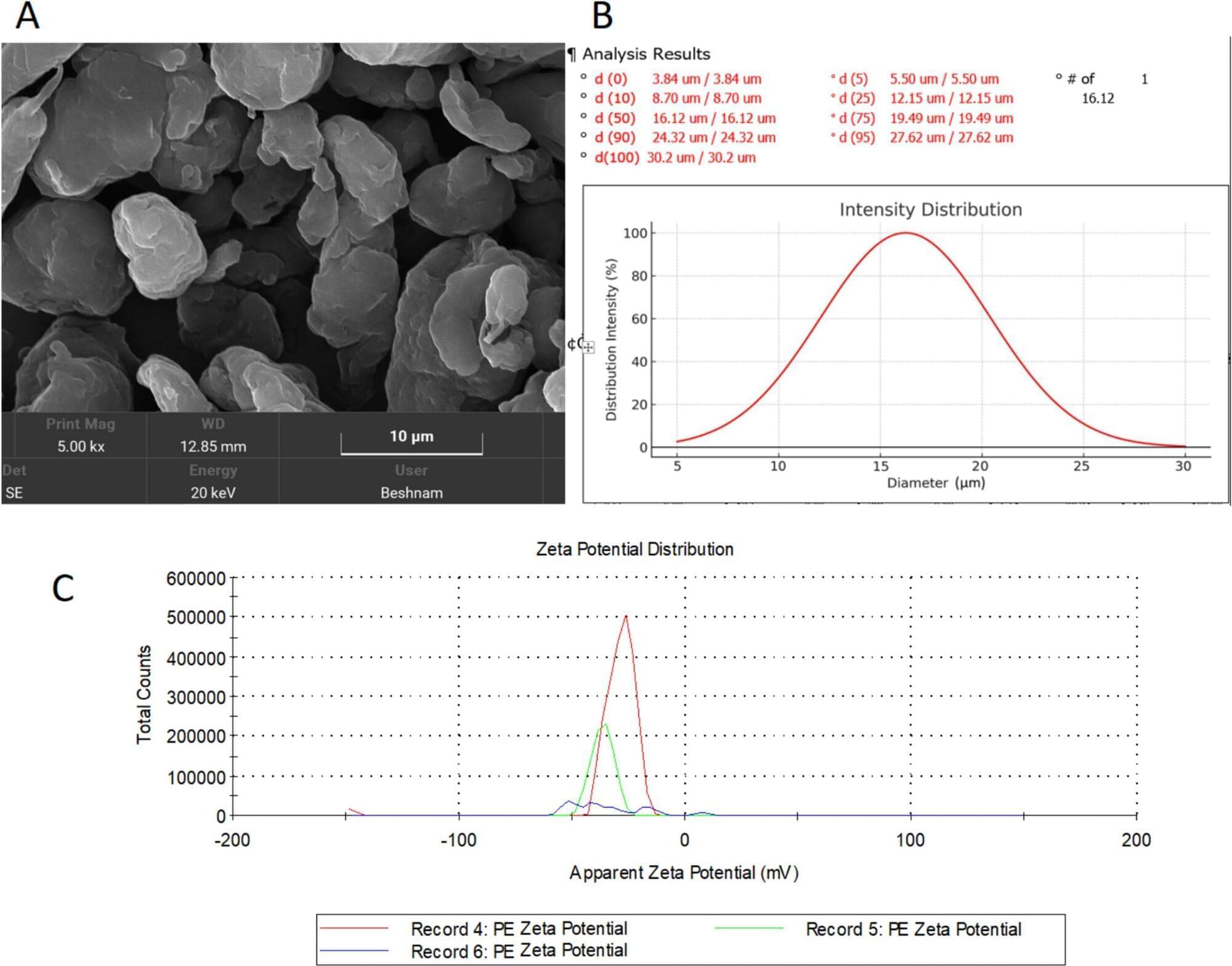
The widespread prevalence of plastics and in particular, microplastics (MPs) raises concerns about their potential toxic effects. MPs, defined as particles smaller than 5 mm, are distributed throughout ecosystem and can enter the human body through the food chain. There is a lack of knowledge regarding MP potential harmful effects on the mammal’s body, especially the brain. This study aimed to examine the impact of low-density polyethylene (LDPE) MPs (< 30 μm) on blood–brain barrier (BBB) integrity, oxidative stress, and neuronal health. Male rats were exposed to LDPE MPs via oral administration for 3 and 6 weeks. The results revealed no significant changes in brain water content across groups. However, BBB integrity was significantly compromised after both 3 and 6 weeks of exposure. Oxidative stress increased in MP-treated groups, evidenced by decreased superoxide dismutase (SOD) levels and elevated malondialdehyde (MDA). Additionally, brain-derived neurotrophic factor (BDNF) levels significantly declined in the 6-week group. Histological analysis indicated neuronal damage and death in both treatment durations. These findings demonstrate that chronic exposure to LDPE MPs impairs BBB integrity, increases oxidative stress, and induces neuronal damage in rats. The results highlight the neurotoxic potential of MPs and emphasize the need for further research to address their possible health risks.
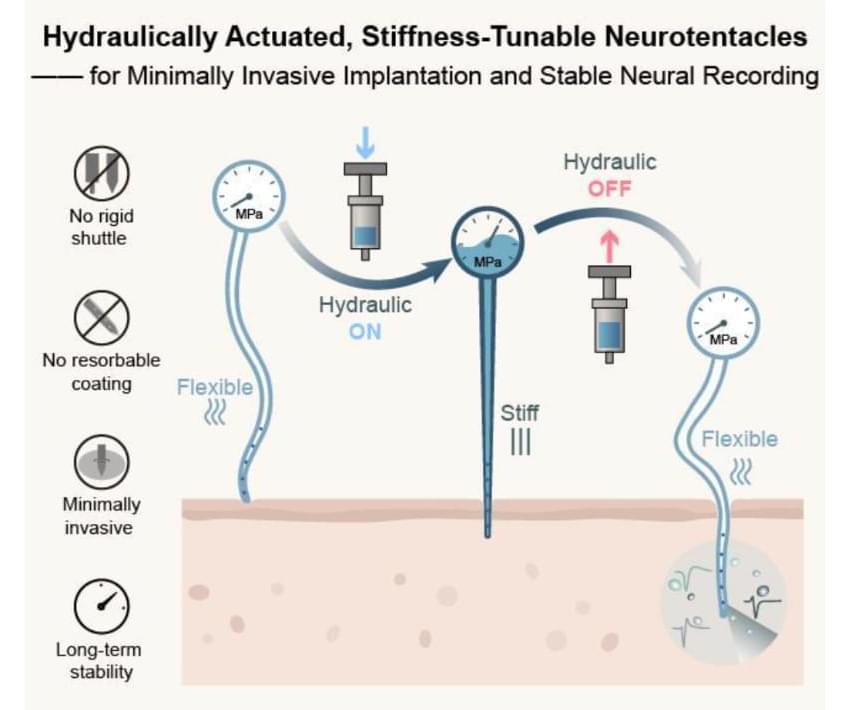
Chinese researchers have made significant progress in developing flexible invasive brain-computer interface implants, creating a stiffness-tunable “Neurotentacle” probe that can reduce implantation damage by 74 percent, Science and Technology Daily reported Tuesday.
The “Neurotentacle” probe developed by researchers at the Institute of Semiconductors, Chinese Academy of Sciences (CAS), contains a tiny hydraulic system. During the implantation, the hydraulically actuated “Neurotentacle” probe stiffens like an inflated balloon to precisely penetrate brain tissue. Once it is in place, it softens afterward to minimize damage and returns to a flexible state to adapt to the brain’s microenvironment, said the report.
The findings were published online in the international journal Advanced Science on July 21.
Make a donation to Closer To Truth to help us continue exploring the world’s deepest questions without the need for paywalls: https://shorturl.at/OnyRq.
Quantum theory is very strange. No act is wholly sure. Everything works by probabilities, described by a wave function. But what is a wavefunction? One theory is that every possibility is in fact a real world of sorts. This is the Many Worlds interpretation of Hugh Everett and what it claims boggles the brain. You can’t imagine how many worlds there would be.
Free access to Closer to Truth’s library of 5,000 videos: http://bit.ly/376lkKN
Watch more interviews on quantum theory: https://bit.ly/3vQwB0f.
David Elieser Deutsch, FRS is a British physicist at the University of Oxford. He is a Visiting Professor in the Department of Atomic and Laser Physics at the Centre for Quantum Computation (CQC) in the Clarendon Laboratory of the University of Oxford.
Register for free at CTT.com for subscriber-only exclusives: http://bit.ly/2GXmFsP
