Long-term stress, measured through hair cortisol, may help predict mental health risks in children living with chronic physical illnesses.


One fundamental feature of neurodegenerative diseases is a breakdown in communication. Even before brain cells die, the delicate machinery that keeps neurons in touch—by clearing away protein waste at the synapses—starts to fail.
When the cleanup falters, the connections between brain cells are impaired and the flow of signals responsible for reasoning, language, memory, and even basic bodily functions are progressively disrupted.
Now, a new study identifies a novel strategy for preventing unwanted proteins from clogging synapses and ultimately congealing into protein plaques.



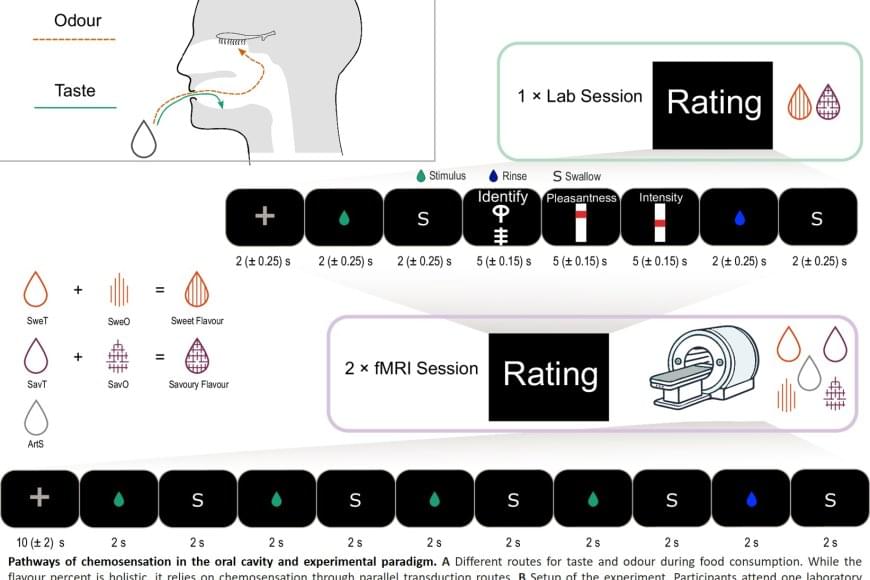
When we eat or drink, we don’t just experience taste, but rather a ‘flavor’. This taste experience arises from a combination of taste and smell, where aromas from food reach the nose via the oral cavity, known as retronasal odor. Researchers have now shown that the brain integrates these signals earlier than previously thought – already in the insula, a brain region known as the taste cortex – before the signals reach the frontal cortex, which controls our emotions and behavior.
“We saw that the taste cortex reacts to taste-associated aromas as if they were real tastes,” explains the lead author. “The finding provides a possible explanation for why we sometimes experience taste from smell alone, for example in flavored waters. This underscores how strongly odors and tastes work together to make food pleasurable, potentially inducing craving and encouraging overeating of certain foods.”
The study involved 25 healthy adults who were first taught to recognize both a sweet taste and a savory taste through combinations of taste and smell. This was followed by two brain imaging sessions using functional magnetic resonance imaging (fMRI), in which the participants were given either a tasteless aroma or a taste without smell. The researchers trained an algorithm to recognize patterns in brain activity for sweet and savory tastes, and then tested whether the same patterns could be identified when the participants were only given aromas.