Psychedelics, a class of psychoactive drugs that typically induce peculiar mental states and hallucinations, are still prohibited for recreational use in most countries worldwide. In recent years, some neuroscientists and medical researchers have been exploring the potential therapeutic effects of these drugs, focusing on the treatment of depression, anxiety and various substance use disorders.
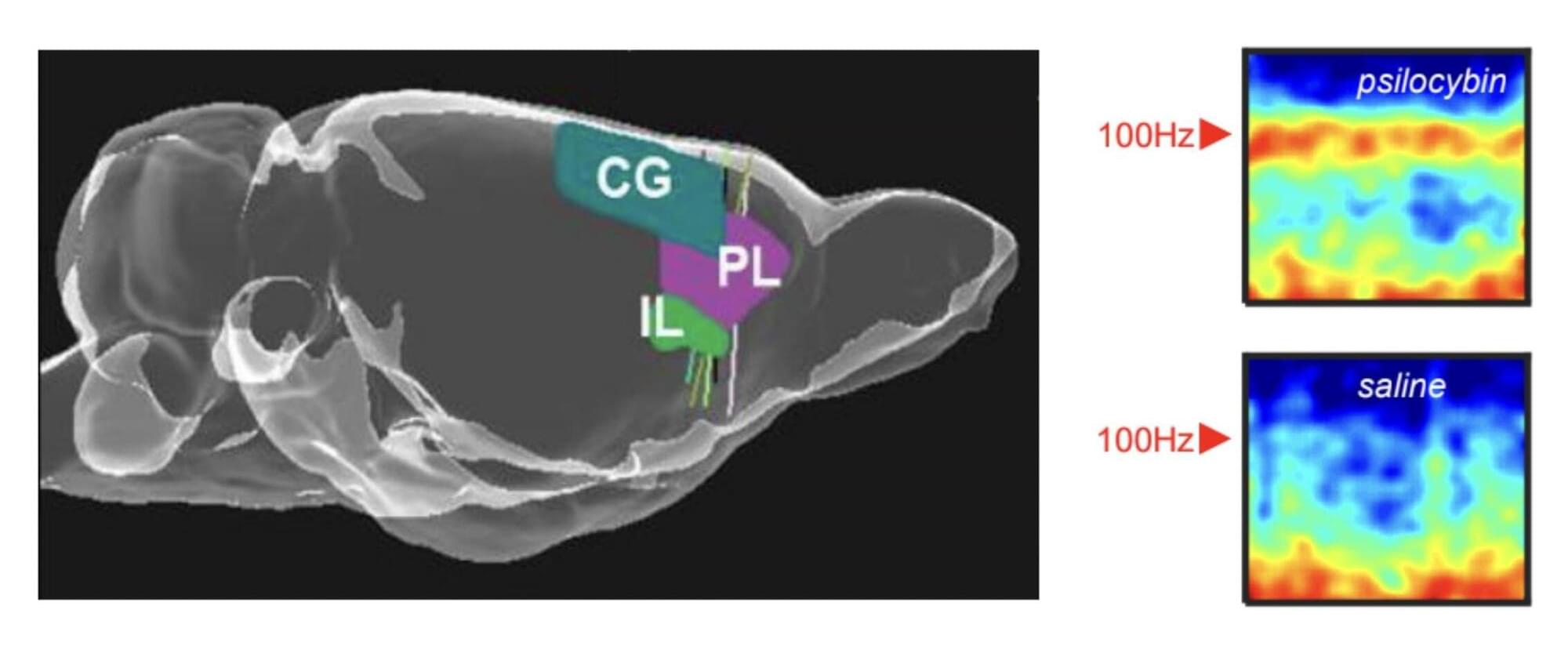
Researchers at the University of Bristol, Compass Pathways plc and other institutes recently carried out a new study involving rats, exploring the effects of the psychedelic compound on the activity of neurons in the medial prefrontal cortex, a brain region that supports decision-making, attention and the regulation of emotions. Their paper, published in Molecular Psychiatry, outlines some of the neural patterns associated with the intake of this compound, which had not yet been observed in human experiments.
“Psychedelic drugs like psilocybin have profound effects on our brains and minds,” Matt Jones, Professor of Neuroscience at the University of Bristol and senior author of the paper, told Medical Xpress. “These effects are fascinating and—as a long history of psychedelic use and recent clinical trials attest—potentially beneficial. This study was driven by two interrelated questions. Firstly, how does a relatively simple, small molecule alter brain activity to completely change our mental model of the world? Secondly, can those effects be harnessed to help treat mental illness?”