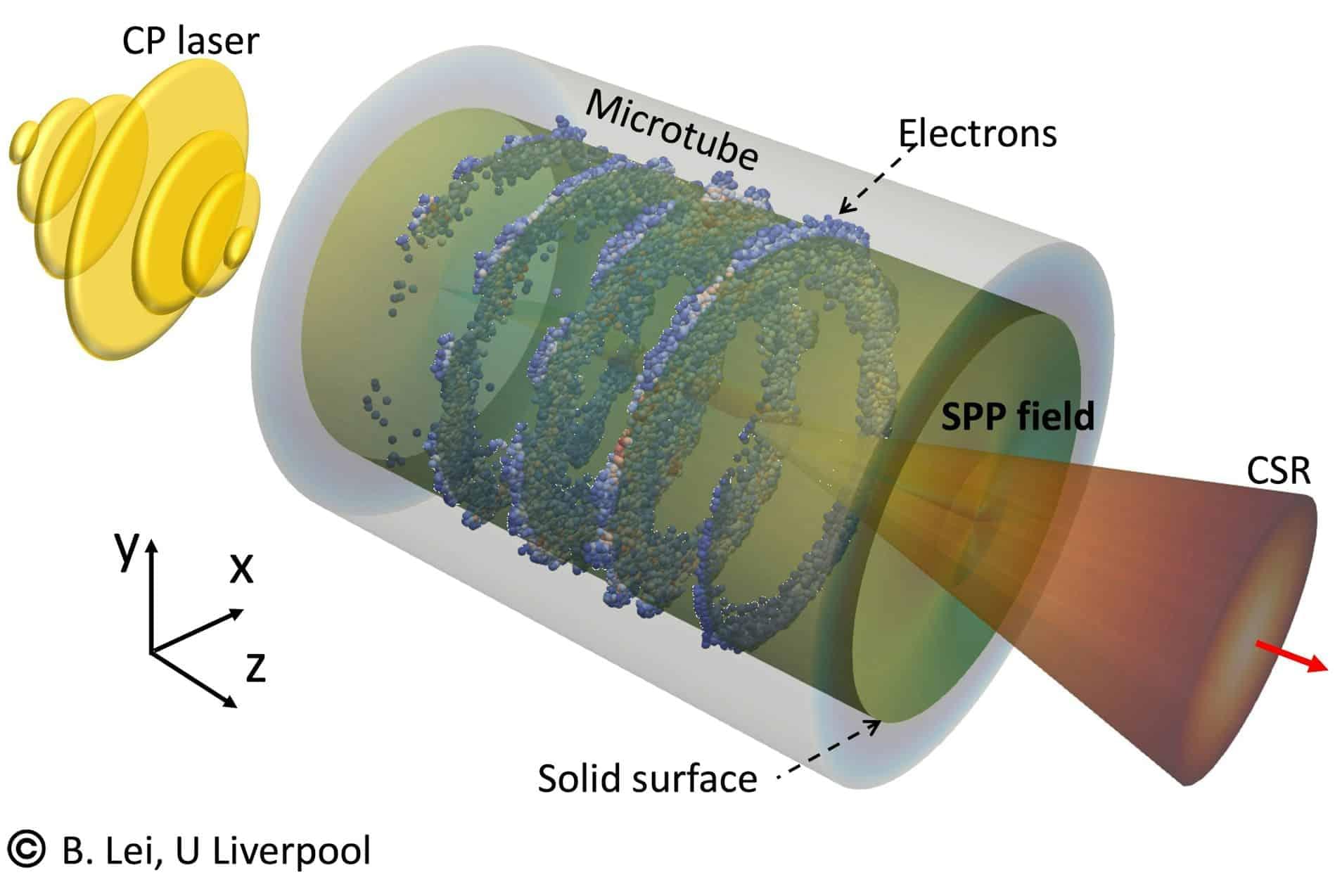
Vehicles and buildings designed to enable survival in extreme environments, such as spacecraft, submarines and sealed shelters, heavily rely on systems for the management of carbon dioxide (CO2). These are technologies that can remove and release CO2, ensuring that the air remains breathable for a long time.
Most existing systems for the capture and release of CO2 consume a lot of energy, as they rely on materials that need to be heated to high temperatures to release the gas again after capturing it. Some engineers have thus been trying to devise more energy-efficient methods to manage CO2 in confined spaces.
Researchers at Guangxi University in China have developed new reconfigurable micro/nano-robots that can reversibly capture CO2 at significantly lower temperatures than currently used carbon management systems.