Motherhood appears to lead to long-lasting increases in gray matter density in the brain, particularly in cognitive and visual areas, which may protect against aging.


Extract from “Evolution, Basal Cognition and Regenerative Medicine”, kindly contributed by Michael Levin in SEMF’s 2023 Interdisciplinary Summer School (http…

What if the secret to longevity wasn’t in the mind or the gut — but in the heart?
Speaking at the inaugural New York Times Well Festival on Wednesday, psychiatrist and researcher Dr. Robert Waldinger announced he and his team were “shocked” by “the biggest predictor of who was going to live long and stay healthy.”
Waldinger, the director of the Harvard Study of Adult Development — the longest-running scientific study of adult life — revealed the predictor was “how connected you were to other people and particularly the warmth of your connection to other people.”
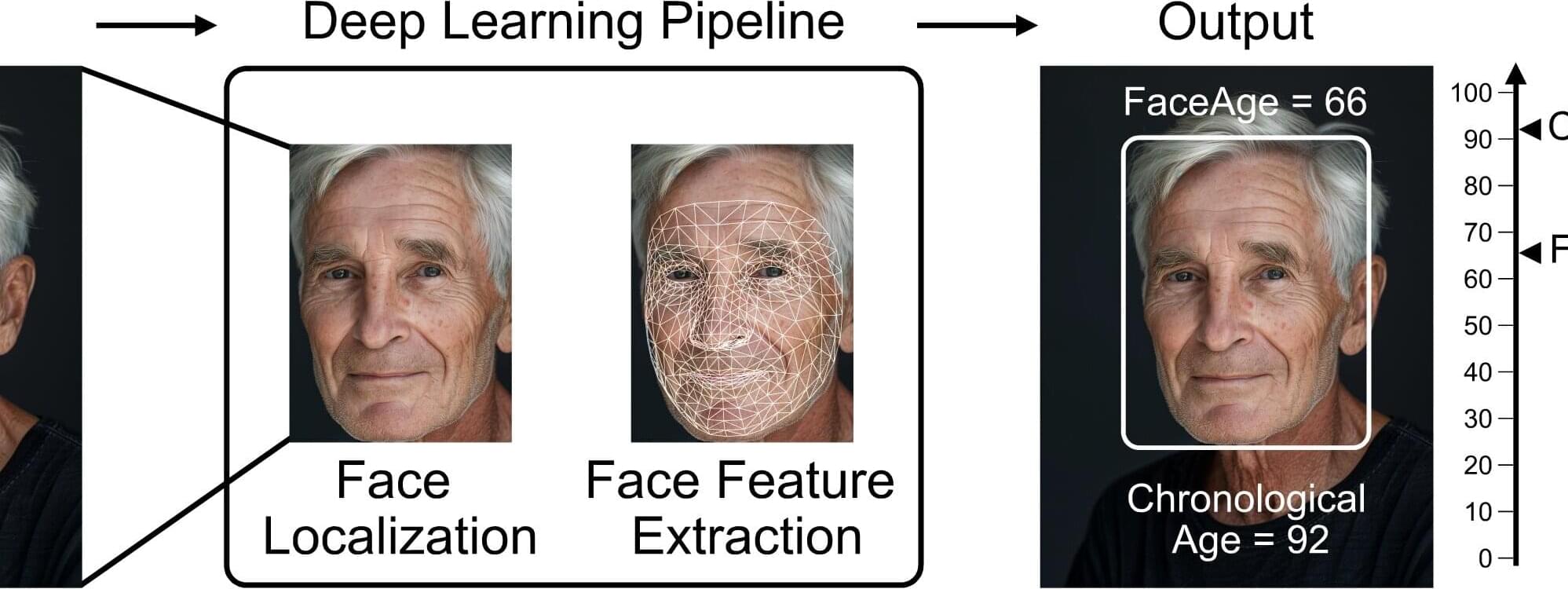
Eyes may be the window to the soul, but a person’s biological age could be reflected in their facial characteristics. Investigators from Mass General Brigham developed a deep learning algorithm called “FaceAge” that uses a photo of a person’s face to predict biological age and survival outcomes for patients with cancer.
They found that patients with cancer, on average, had a higher FaceAge than those without and appeared about five years older than their chronological age.
Older FaceAge predictions were associated with worse overall survival outcomes across multiple cancer types. They also found that FaceAge outperformed clinicians in predicting short-term life expectancies of patients receiving palliative radiotherapy.

Meridian Magazine positions itself as a publication for members of The Church of Jesus Christ of Latter-day Saints, the largest Mormon denomination. I don’t know much about Meridian or the people behind it. But today I learned that they’re willing to publish a fear-mongering distortion of Transhumanism, “Human 2.0 Is Here — And You Didn’t Even Notice” by Alexis Tarkaleson. Despite their positioning, I wish to make make clear that such behavior is not aligned with the values that the Church advocates.
Tarkaleson says “mind uploading” is an outlandish tale. What’s her take on tales of transfiguration and resurrection? Are those equally outlandish? Surely she’s aware that those doctrines require the possibility of mind (or spirit body) moving from one physical body to another, consistent with hypotheses of mind uploading.
How about cryonics, yet another outlandish tale she identifies? I’m curious to know what she thinks about the Church’s advocacy to collect genealogy and preserve family history, with intent to facilitate redemption of the dead. And what about proxy rituals that we perform for the dead? Most of the world probably thinks the Church’s practices in these areas are at least as outlandish as those of cryonicists.

A selfie can be used as a tool to help doctors determine a patient’s “biological age” and judge how well they may respond to cancer treatment, a new study suggests.
Because humans age at “different rates” their physical appearance may help give insights into their so-called “biological age” – how old a person is physiologically, academics said.
The new FaceAge AI tool can estimate a person’s biological age, as opposed to their actual age, by scanning an image of their face, a new study found.

So size does matter?
Mammal’s lifespans linked to brain size and immune system function, says new study.
The researchers looked at the maximum lifespan potential of 46 species of mammals and mapped the genes shared across these species. The maximum lifespan potential (MLSP) is the longest ever recorded lifespan of a species, rather than the average lifespan, which is affected by factors such as predation and availability of food and other resources.
The researchers, publishing in the journal Scientific Reports, found that longer-lived species had a greater number of genes belonging to the gene families connected to the immune system, suggesting this as a major mechanism driving the evolution of longer lifespans across mammals.
For example, dolphins and whales, with relatively large brains have maximum lifespans of 39 and up to 100 years respectively, those with smaller brains like mice, may only live one or two years.
However, there were some species, such as mole rats, that bucked this trend, living up to 20 years despite their smaller brains. Bats also lived longer than would be expected given their small brains, but when their genomes were analysed, both these species had more genes associated with the immune system.
The results suggest that the immune system is central to sustaining longer life, probably by removing aging and damaged cells, controlling infections and preventing tumour formation.
Hypertension and other health risks accelerate brain aging, as shown in a 16-year study using MRI data and predictive modeling.
Chinese scientists have conducted a population-based cohort study to examine the long-term impact of unhealthy lifestyles, metabolic abnormalities, and other risk factors on brain aging. The findings showed that these factors significantly accelerate brain aging, and the researchers proposed strategies to support brain health. Their study was published in Research.
Background.
An international study led by the Institut de Neurociències at the UAB (INc-UAB) has shown that increasing levels of the Klotho protein in mice extends lifespan and improves both physical and cognitive health when aging.
As we grow older, it is natural to lose muscle and bone mass, leading to greater frailty and a higher risk of falls and serious injuries. Cognitively, neurons progressively degenerate and lose connections, while diseases such as Alzheimer’s and Parkinson’s become more prevalent. In a society where the population is steadily aging, reducing these effects is one of the main challenges for research.
Now, in an article published in Molecular Therapy, an international research team led by Professor Miguel Chillón, ICREA researcher at the INc-UAB, has shown that increasing levels of the secreted form of the Klotho protein (s-KL) improves aging in mice.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/