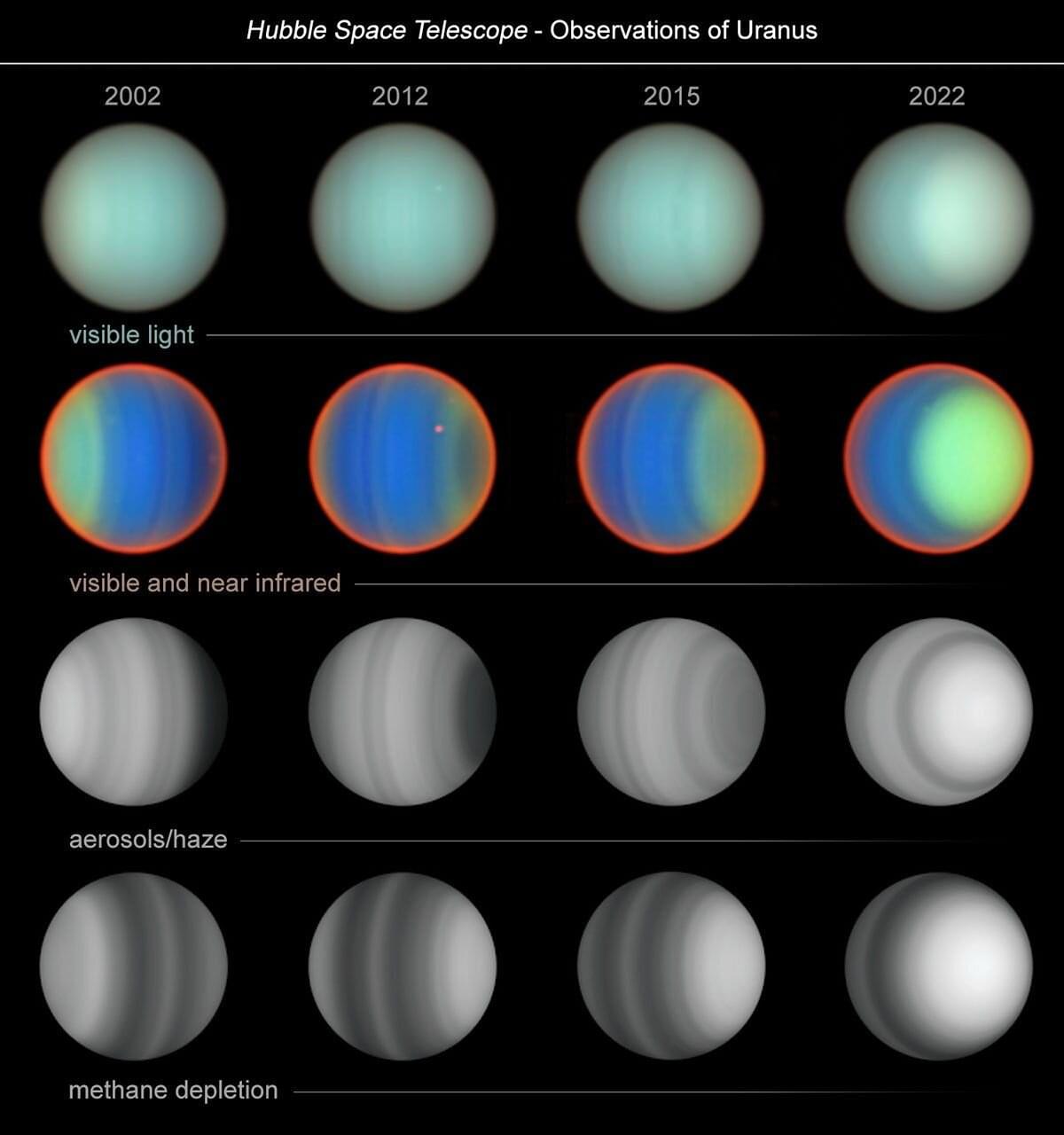
The ice-giant planet Uranus, which travels around the sun tipped on its side, is a weird and mysterious world. Now, in an unprecedented study spanning two decades, researchers using NASA’s Hubble Space Telescope have uncovered new insights into the planet’s atmospheric composition and dynamics. This was possible only because of Hubble’s sharp resolution, spectral capabilities, and longevity.
The team’s results will help astronomers to better understand how the atmosphere of Uranus works and responds to changing sunlight. These long-term observations provide valuable data for understanding the atmospheric dynamics of this distant ice giant, which can serve as a proxy for studying exoplanets of similar size and composition.
When Voyager 2 flew past Uranus in 1986, it provided a close-up snapshot of the sideways planet. What it saw resembled a bland, blue-green billiard ball. By comparison, Hubble chronicled a 20-year story of seasonal changes from 2002 to 2022. Over that period, a team led by Erich Karkoschka of the University of Arizona, and Larry Sromovsky and Pat Fry from the University of Wisconsin used the same Hubble instrument, STIS (the Space Telescope Imaging Spectrograph), to paint an accurate picture of the atmospheric structure of Uranus.