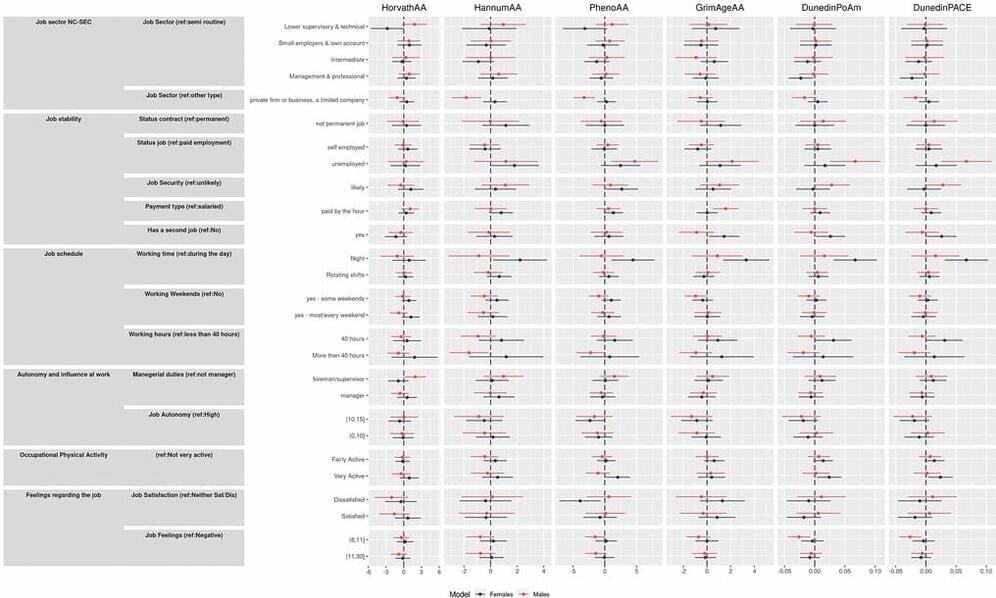
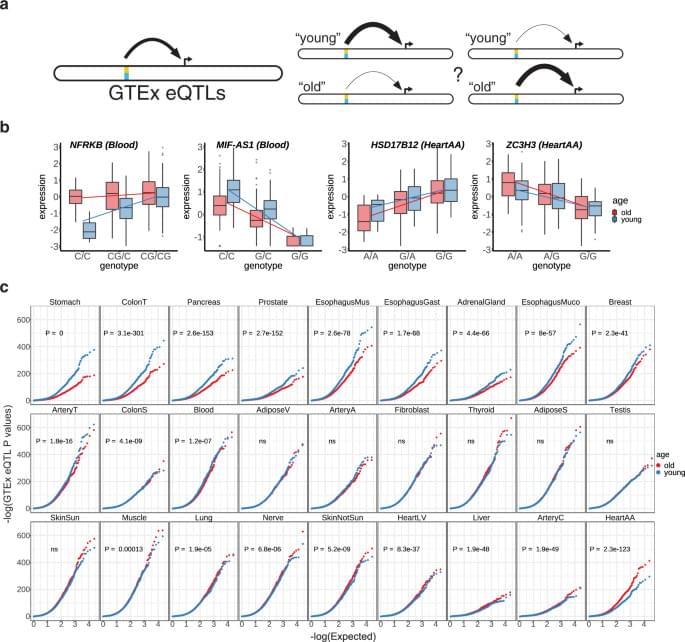
(http://www.pharma.unizg.hr/en/about-us/staff/gordan–lauc, 450.html) is Professor of Biochemistry and Molecular Biology at the University of Zagreb, Faculty of Pharmacy and Biochemistry, and Founder and CEO of Genos Ltd. (https://genos-glyco.com/), a research-intensive SME located in Zagreb, Croatia with core of expertise in molecular genetics and glycomics (The comprehensive study the entire complement of sugars, whether free or present in more complex molecules of an organism) and they perform contract research, contract analysis and service for numerous universities, hospitals and private individuals in Europe and overseas.
Prof. Dr. Lauc also is CSO of GlycanAge LTD (https://glycanage.com/), a company that has developed a ground-breaking test that analyses your personal glycobiome for insights in improving your health and monitoring your biological age, and Co-Director of the Human Glycome Project (https://human-glycome.org/).
Prof. Dr. Lauc graduated with a degree in molecular biology at the University of Zagreb Faculty of Science in 1992, and obtained Ph.D. in Biochemistry and the University of Zagreb in 1995. He got his postdoctoral training at the Institute for Medical Physics and Biophysics in Münster and Johns Hopkins University in Baltimore. Since 1993 he has been employed at the Faculty of Pharmacy and Biochemistry in Zagreb. Between 1998 and 2010 he was also part-time employed at the University of Osijek School of Medicine where he founded a DNA laboratory for the identification of war victims and also served as Vice-Dean for Science between 2001 and 2005.
Prof. Dr. Lauc is author of over 100 research papers published in international journals and six international patents. He was invited to lecture at numerous international conferences, elected for visiting professor at the Johns Hopkins University and in 2011 also inducted in the prestigious Johns Hopkins Society of Scholars. If 2012 he was appointed Honorary Professor at the University of Edinburgh and Adjunct Professor at the Edith Cowan University in Perth.
Prof. Dr. Lauc chaired a number of conferences, including the “European Science Foundation Exploratory Workshop on Glycoscience” which resulted in the creation of the “European Glycoscience Forum”.
Prof. Dr. Lauc was a chairman of the committee that prepared Croatian National Action plan for the increased investment in research in development (2007), and was a member of the National Science Council between 2009 and 2013 and also and President of the National Council for Natural Sciences. He is a President-elect of the International Glycoscience Organization and member of the Steering Committee of the European Glycoscience Forum.