Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhDDiscount Links: Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7x…
Category: life extension – Page 155
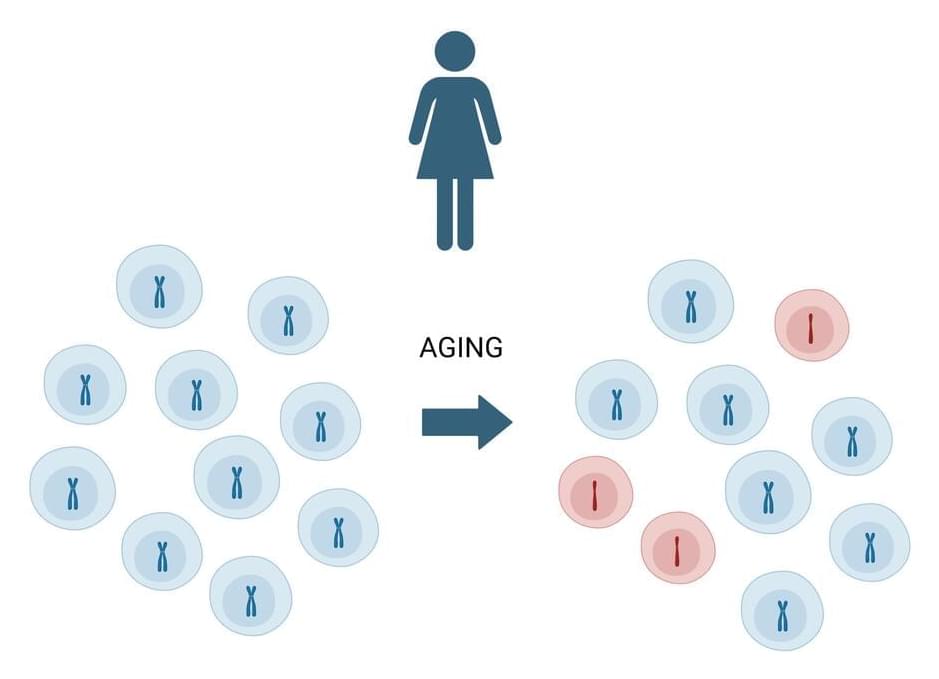

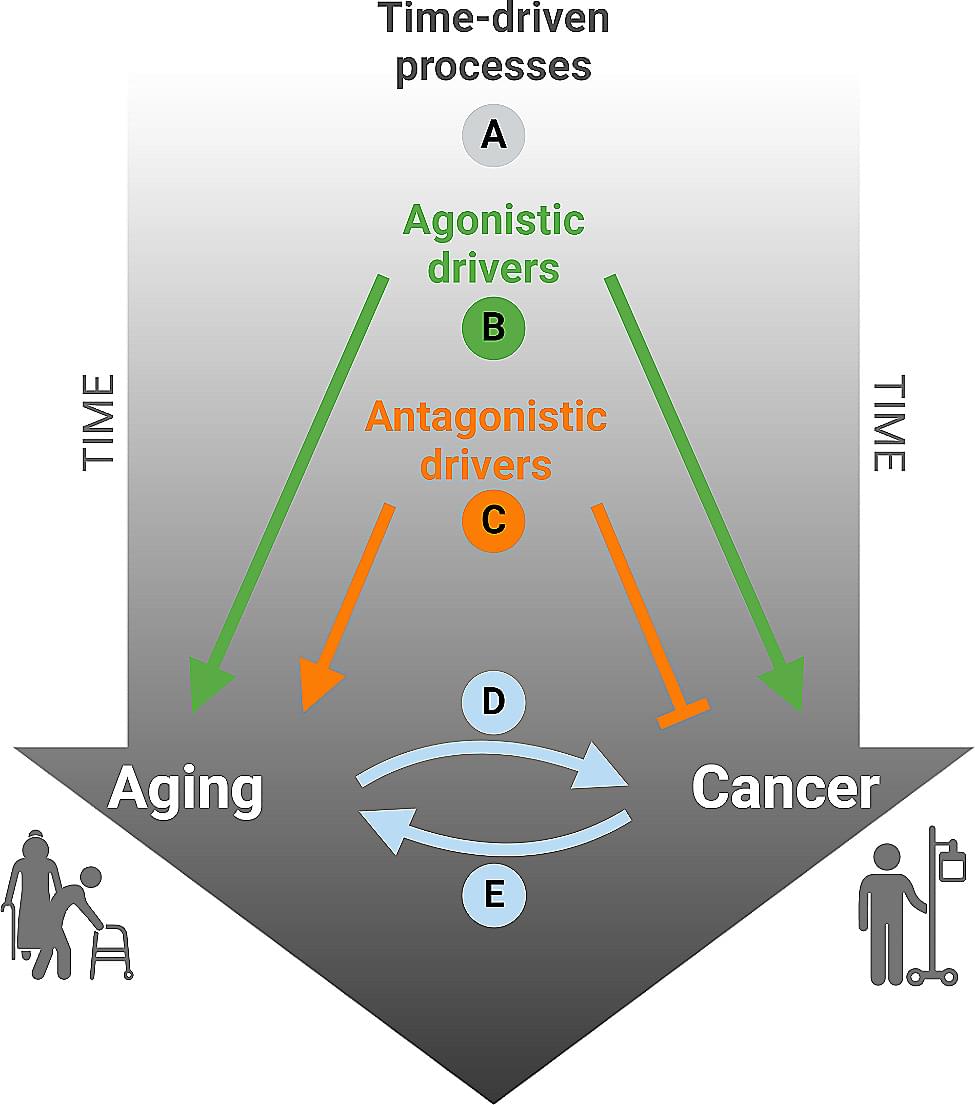
Aging and cancer
Exhibit apparent links that we will examine in this review. The null hypothesis that aging and cancer coincide because both are driven by time, irrespective of the precise causes, can be confronted with the idea that aging and cancer share common mechanistic grounds that are referred to as ‘hallmarks’. Indeed, several hallmarks of aging also contribute to carcinogenesis and tumor progression, but some of the molecular and cellular characteristics of aging may also reduce the probability of developing lethal cancer, perhaps explaining why very old age ( 90 years) is accompanied by a reduced incidence of neoplastic diseases. We will also discuss the possibility that the aging process itself causes cancer, meaning that the time-dependent degradation of cellular and supracellular functions that accompanies aging produces cancer as a byproduct or ‘age-associated disease’
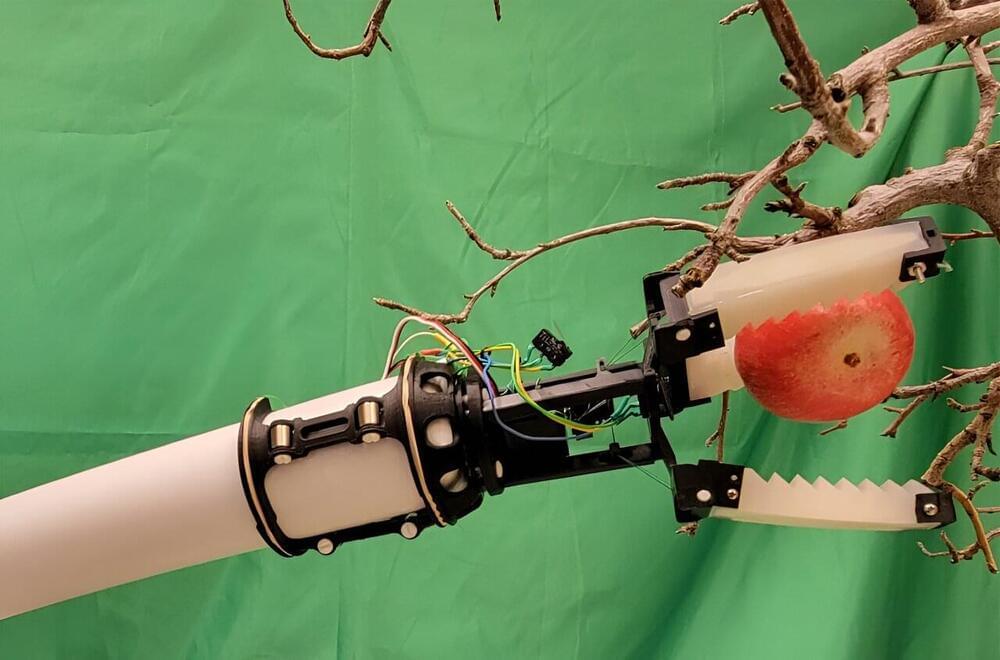
New robotic gripper for automated apple picking developed
Washington state leads the nation in apple production, and in 2022, the industry contributed more than two billion dollars to the U.S. gross domestic product. Throughout Washington, farms employ anywhere from a dozen to hundreds of workers each year for orchard operations, including for pollination, pruning, flower thinning and fruit harvesting. With an aging population and a decrease in migrant farm workers, however, farmers have struggled to meet their needs for workers during harvest season.
In recent years, researchers have started developing robotic apple harvesting systems, but the ones that have been developed are expensive and complex to use in orchards.
Ninatanta, who grew up in Yakima, Washington, picked fruit alongside his parents during his childhood. When he began his work with Luo on a robotic apple gripper, he had his parents videotape their work, so he could model his gripper on their handiwork.
New neuroscience research reveals the remarkable impact of exercise on brain cells
The study offers promising evidence that exercise can counteract age-related changes in the brain, particularly by rejuvenating microglia. The findings contribute to our understanding of how physical activity can benefit cognitive health and open up new avenues for developing interventions to prevent or slow cognitive decline during aging.
“One of the goals is it to encourage elderly to exercise as we have demonstrated that it is possible to reverse some of the negative aspect of ageing on the brain and thereby improve cognitive performance,” Vukovic said. “The other long-term goals is to find ways and treatments to help elicit the beneficial aspect of exercise on the brain in those individual that are unable to exercise or bed-bound.”
The study, “Exercise rejuvenates microglia and reverses T cell accumulation in the aged female mouse brain,” was authored by Solal Chauquet, Emily F. Willis, Laura Grice, Samuel B. R. Harley, Joseph E. Powell, Naomi R. Wray, Quan Nguyen, Marc J. Ruitenberg, Sonia Shah, and Jana Vukovic.

Creativity and Humor shown to Promote Well-Being in Older Adults via Similar Mechanisms
Many people associate aging with a decline in cognitive function, health issues, and reduced activity. Uncovering mental processes that can boost the well-being of the older adults could be highly beneficial, as it could help to devise more effective activities aimed at improving their quality of life.
Researchers at University of Brescia and the Catholic University of the Sacred Heart recently carried out a study investigating the contribution of creativity and humor to the well-being of the elderly. Their findings, published in Neuroscience Letters, show that these two distinct human experiences share common psychological and neurobiological processes that promote well-being in older adults.
“Our recent study belongs to a line of research aimed at investigating the cognitive resources which are still available to elderly people and at understanding how such resources can support well-being,” Alessandro Antonietti, co-author of the paper, told Medical Xpress.

A Portal to the Past: Hubble Reveals an Ancient Witness to a Galactic Merger
NGC 2005, a globular cluster in the Large Magellanic Cloud, serves as a crucial piece of evidence supporting the theory of galaxies evolving through mergers.
This mesmerizing image from the Hubble Space Telescope features the globular cluster NGC 2005. While it is not unusual in and of itself, it is a peculiarity in relation to its surroundings.
NGC 2005 is located about 750 light-years from the heart of the Large Magellanic Cloud (LMC), which is the Milky Way ’s largest satellite galaxy and which itself lies about 162,000 light-years from Earth. Globular clusters are densely packed clusters that can constitute tens of thousands or millions of stars. Their density means that they are tightly gravitationally bound and are therefore very stable. This stability contributes to their longevity: globular clusters can be billions of years old, and as such often comprise very old stars.