“Information and the Nature of Reality: From Physics To Metaphysics” by Paul Davies and Niels Henrik Gregersen Book Link: https://amzn.to/41GMVl6 (Affiliate link: If you use this link to buy something, I may earn a commission at no extra cost to you.) Playlist: • Two AI’s Discuss: The Quantum Physics… This collection of essays, “Information and the Nature of Reality,” explores the evolving role of information from physics to metaphysics. It examines how the concept of matter has shifted historically, particularly with advances in quantum physics, and considers materialism’s limitations as a worldview. The texts propose that information may be as fundamental as matter and energy in understanding the universe, investigating its influence on biology, consciousness, and computation. Several contributions consider the theological implications, pondering God as an ultimate source of information and discussing the relationship between divine action and natural processes. Ultimately, the text argues for interdisciplinary dialogue between science, philosophy, and theology to form an adequate theory of the natural world. • Paul Davies and Niels Henrik Gregersen in the introduction introduce the central question of whether information matters in understanding reality, setting the stage for the book’s interdisciplinary exploration. • Ernan McMullin traces the historical evolution of the concept of matter in philosophy and its relationship to physics in his essay. • Philip Clayton in his contribution, Unsolved dilemmas: the concept of matter in the history of philosophy and in contemporary physics, explores the persistent challenges and transformations in understanding matter across philosophical history and modern physics. • Paul Davies in Universe from bit discusses the idea that the universe may fundamentally be based on information. • Seth Lloyd in The computational universe presents the concept of the universe as a vast quantum computer. • Henry Stapp in Minds and values in the quantum universe examines the role of consciousness and values within the framework of quantum mechanics. • John Maynard Smith in The concept of information in biology investigates the application and implications of information concepts within biological systems, particularly in genetics and evolution. • Terrence W. Deacon in What is missing from theories of information? argues that the crucial aspect of information is its inherent reference to something absent. • Bernd-Olaf Küppers in Information and communication in living matter explores the semantic dimensions of information and its fundamental role in biological processes. • Jesper Hoffmeyer in Semiotic freedom: an emerging force proposes a biosemiotic perspective, emphasizing the importance of signs and interpretation in understanding life. • Holmes Rolston, III in Care on Earth: generating informed concern examines the evolutionary basis and significance of caring and concern in the natural world. • Arthur Peacocke in The sciences of complexity: a new theological resource? explores how the sciences of complexity can offer new insights for theological understanding. • Keith Ward in God as the ultimate informational principle posits that God can be understood as the fundamental source and sustainer of information in the universe. • John F. Haught in Information, theology, and the universe explores the relationship between information, theology, and our understanding of the cosmos. • Niels Henrik Gregersen in God, matter, and information: towards a Stoicizing Logos Christology proposes a theological framework that integrates the concepts of God, matter, and information, drawing on Stoic philosophy and Christian theology. #InformationReality #PhysicsMetaphysics #NatureOfReality #QuantumInformation #BiologicalInformation #PhilosophyOfScience #ScienceAndTheology #CosmicInformation #OriginOfLife #UltimateReality #MeaningOfInformation #ComplexSystems #HistoryOfScience #Interdisciplinary #SciencePhilosophy #deepdive #skeptic #podcast #synopsis #books #bookreview #ai #artificialintelligence #booktube #aigenerated #history #alternativehistory #aideepdive #ancientmysteries #hiddenhistory #futurism #videoessay
Category: genetics – Page 91
The Future of Human Evolution: AI, Genetic Engineering, and the Rise of Post-Human Civilization
“The Future of Human Evolution: AI, Genetic Engineering, and the Rise of Post-Human Civilization”
What happens when human evolution is no longer shaped by nature but by artificial intelligence and genetic engineering? This story explores the rise of AI-enhanced humans in a futuristic medieval world, where the fusion of bioengineering, AI consciousness, and neural implants creates a post-human era. As civilizations embrace transhumanism, traditional humanity faces extinction, replaced by a new species of synthetic life. Will this AI-driven society achieve ultimate enlightenment, or will it lose the essence of what makes us human?
The battle between future civilization, advanced technology, and those clinging to the past intensifies as digital immortality reshapes the meaning of existence. This cybernetic future forces us to question our identity—can genetic modification and AI singularity coexist with the soul of humanity? Witness the evolution of intelligence, the struggle between AI vs humanity, and the uncertain fate of a world where consciousness itself is no longer biological.
0:00 — Introduction: The Future of Human Evolution.
8:25 — AI & Genetic Engineering: Unlocking Human Potential.
16:50 — Ethical Dilemmas of Genetic Modification.
25:15 — The Rise of Engineered Intelligence.
33:40 — Genetic Enhancements & Social Stratification.
42:05 — AI in Education, Work, and Society.
50:30 — The Quest for Longevity & Immortality.
58:55 — Resistance Movements Against Enhancement.
1:07:20 — The First AI-Integrated Humans.
1:15:45 — The Breakdown of Traditional Humanity.
1:24:10 — Post-Human Civilizations & Digital Consciousness.
1:32:35 — The Divide Between Organic & Artificial Life.
1:41:00 — The Singularity & The End of Natural Evolution.
1:49:25 — What Comes After Humanity?
Sources.
Bostrom, N. (2014). Superintelligence: Paths, Dangers, Strategies. Oxford University Press.
Harari, Y. N. (2017). Homo Deus: A Brief History of Tomorrow. Harper.
Kurzweil, R. (2005). The Singularity Is Near: When Humans Transcend Biology. Penguin.
Tegmark, M. (2017). Life 3.0: Being Human in the Age of Artificial Intelligence. Knopf.
Goertzel, B. (2020). Artificial General Intelligence: Concept, State of the Art, and Future Directions. Springer.
#FutureOfHumanity #AIandGenetics #PostHumanEra #ArtificialEvolution #CyberneticFuture.
YOU MAY LIKE
Individual and additive effects of vitamin D, omega-3 and exercise on DNA methylation clocks of biological aging in older adults from the DO-HEALTH trial
Longevity Snapshot #5 — Reviewing a trial which shows that a combination of Omega 3, Vitamin D and Exercise slows down biological aging in older adults according to 4 epigenetic clocks.
Applying epigenetic clocks to samples from the DO-HEALTH trial, Bischoff-Ferrari et al. report a small protective effect of omega-3 supplementation over 3 years on several clocks and an additive protective effect of omega-3, vitamin D and exercise using PhenoAge.

Violence Leaves Its Mark on Our Genes For Generations, Study Finds
A stressful life can leave marks on our genetic code, some of which can even be passed on to our children. A study now reveals how the biological impact of trauma on a mother persists long after the violent acts themselves have passed.
The international team of researchers demonstrate the physical mechanisms behind intergenerational trauma in humans, explaining why people with a family history of adversity are more prone to mental health conditions like anxiety and depression, despite not having experienced the adverse events themselves.
The researchers analyzed DNA collected from 48 Syrian families across three generations. These families included grandmothers or mothers who while pregnant had fled the 1982 siege and massacre in Hama or the 2011 armed uprising – both part of the ongoing Syrian civil war.

Who gets ownership of useful genetic data?
Cow D lived on a dairy farm in New Zealand. The animal looked like the typical black-and-white cow farmers raise for milk, except for one thing: Researchers had outfitted Cow D with an artificial fistula—a hole offering them a way to reach the microbes inhabiting the animal’s bathtub-size stomach. But it’s what happened next that offers a porthole into the global debate over the use of genetic data.
In the spring of 2009, Samantha Noel, then a doctoral researcher at Massey University in Palmerston North, New Zealand, reached into Cow D’s rumen and plucked out a strain of Lachnospiraceae bacterium, later dubbed ND2006. Another team of geneticists sequenced the microbe’s complete set of genes, or genome, and uploaded the information, which was then shared with GenBank, a public database run by the US National Institutes of Health. If genes are the book of life, then this process was like adding a digital copy to an online library. In policy circles, these lines of code go by another name: digital sequence information, or DSI.
James Giordano ASO
The future of warfare starts in your mind. Understand how Neuroscience, Technology/AI and the OODA loop affects your flow. The world is changing, and cognitive warfare is at the forefront. In our latest podcast episode, we sit down with James Giordano, PhD, a Navy veteran and an expert in neurocognitive science, to delve into the world of cognitive warfare.
Stay in the Loop: https://www.aglx.com/newsletter-signup-north-america.
From the impact of emotions on decision-making to the integration of artificial intelligence and human cognition, this episode challenges your perspective on the battlefield. Join us as we explore the ethical implications of genetic modifications, the transformative effects of psychedelics, and the complexities of data usage in the digital age. Get ready to reimagine the relationship between technology, culture, and language. Don’t miss out on this opportunity to gain valuable insights from our thought-provoking conversation with Dr. Giordano. Tune in now to stay ahead of the curve on the evolving landscape of warfare!
00:00 — Understanding the OODA loop: A Neuroscience Perspective.
09:11 — Exploring Fifth Generation Warfare and Liminal Warfare.
16:06 — The Long Game: China’s Strategic Plan.
22:19 — Understanding Cognitive Warfare and Human-Machine Teaming.
25:52 — The Evolution of Human-Machine Teaming.
29:11 — Human Involvement in AI Decision Making.
36:01 — The Ethics of Paternalistic AI Systems.
40:43 — Technology’s Impact on Cognitive Engagement.
45:13 — Exploring Technologies for Human Performance Enhancement.
55:59 — Diving Into Attacking Mode and Ethics.
56:24 — Hacking the Human Genome.
59:37 — Epigenetic Modification and Phenotypic Shift.
1:04:54 — The Psychedelic Revolution.
1:11:18 — Revisiting Alcohol and Caffeine: Benefits and Burdens.
1:19:18 — Impact of Technology on Cognitive Capacity.
1:23:33 — Information Overload and Burdens.
1:27:02 — Ownership and Security of Personal Data.
1:31:56 — Identifying Predispositional Traits.
1:33:49 — Data Manipulation and Biometrics.
1:40:13 — Cultural Impact of Technology.
1:48:55 — The Role of Education in Integrating Science, Technology, Ethics, and Policy.
1:54:30 — Major Threats and Concerns in Today’s World.
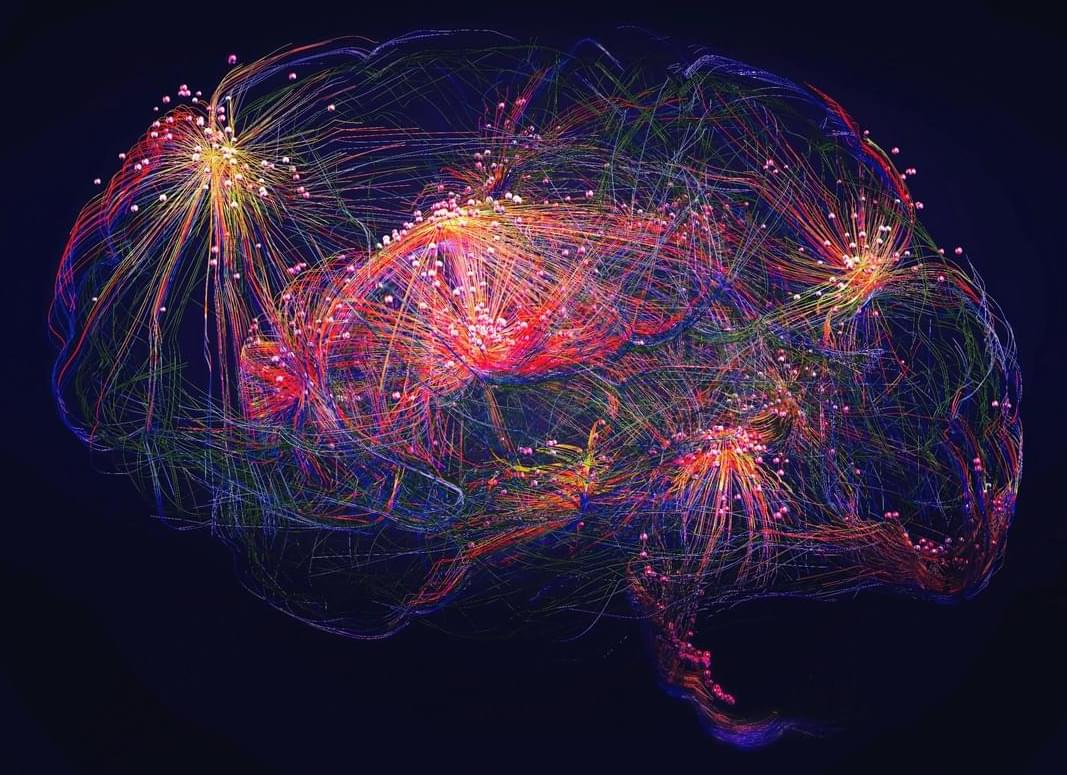
Unraveling the brain’s hidden motor modules
Vertical columns How the Brain Maps Jaw Movements: A Hidden Architecture of Motion.
Our brains contain intricate maps that guide every voluntary movement we make, from reaching out to grab a cup to the delicate motions involved in speaking or chewing. But how exactly are these maps organized, and what role do different types of brain cells play in shaping them?
A new study dives deep into the orofacial motor maps—the brain’s blueprint for controlling jaw movements—revealing a surprising level of organization. Researchers used optogenetics, a technique that activates specific neurons with light, to map out how different classes of excitatory neurons contribute to jaw motion in mice. What they found was remarkable: rather than a single unified map, the motor cortex is divided into distinct, genetically defined modules, each governing jaw movement from different brain regions, including sensory, motor, and premotor areas.
These modules don’t act in isolation. When one was stimulated, activity rippled across the brain, converging in the primary motor cortex, the region that directly controls movement. What’s more, when the mice learned new motor skills—such as refining their licking motion—some of these modules expanded, adapting to support the learned behavior.
This research suggests that voluntary movement isn’t just dictated by a single command center. Instead, a network of specialized cell groups collaborates across different parts of the brain, dynamically adjusting as we learn new motor skills. Understanding this fine-tuned motor map could have implications for treating movement disorders or even advancing brain-computer interfaces in the future.
Scientists have identified previously unknown neural modules in the brain that control movement and adapt during skill learning. Their findings challenge long-held ideas about how the brain organizes movement.
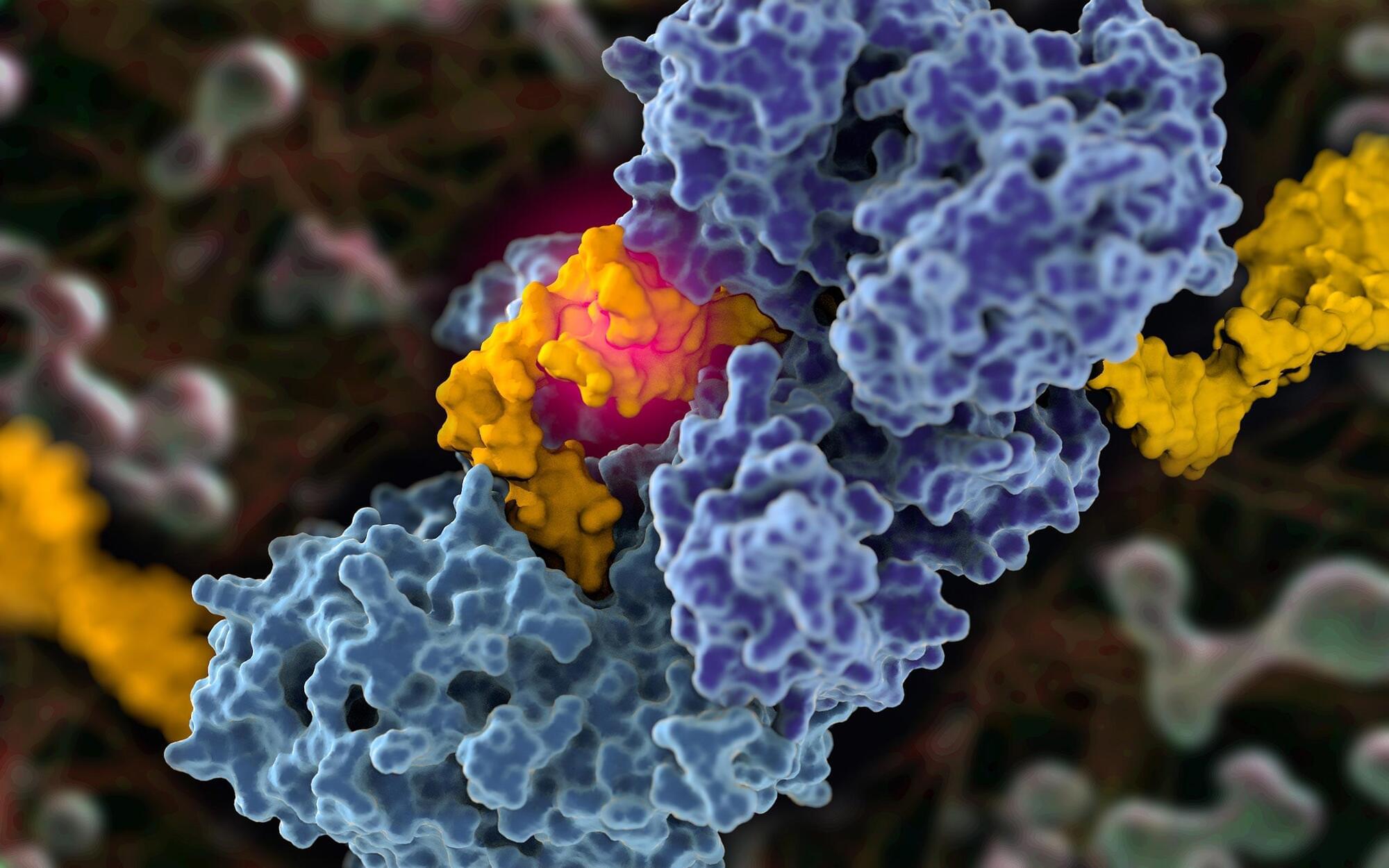
Scientists Capture First-Ever Images of Cancer’s Covert DNA Repair Strategy
A new structural blueprint paves the way for improved targeting of cancer cells, particularly those with BRCA1 and BRCA2 mutations. DNA repair proteins function as the body’s molecular editors, continuously identifying and correcting damage to our genetic code. A longstanding challenge in cancer research has been understanding how cancer cells exploit one such protein—polymerase theta (Pol-theta)—to support their survival. Now, scientists at Scripps Research have captured the first high-resolution images of Pol-theta in action, shedding light on its role in cancer development.

Inside the scientific quest to reverse human aging
Superlongevity via epigenetic reprogramming. 🏆
Life Biosciences:
“If the FDA approves its application, the company will repeat the methods from the mouse and monkey experiments, Rosenzweig-Lipson said. Scientists will inject volunteers’ eyes with Yamanaka factors that can be turned off or on with the antibiotic doxycycline, Rosenzweig-Lipson said. The hope is that the cells in people’s damaged optic nerves will grow more youthful at an epigenetic level, and their vision will improve.”
Can reprogramming our genes make us young again? A breakthrough in longevity research may be nearing its first human trials.

The secret of how Greenland sharks can live for centuries without getting cancer
The eye protein rhodopsin of the Greenland shark was found to have amino acid variations that made them more adept at processing blue-light wavelengths – a feature that is advantageous when living in the dim deep ocean waters.
“These genomic analyses offer new insights into the molecular basis of the exceptional longevity of the Greenland shark and highlight potential genetic mechanisms that could inform future research into longevity,” scientists wrote in the study.