The Investor Relations website contains information about Eli Lilly and Company’s business for stockholders, potential investors, and financial analysts.


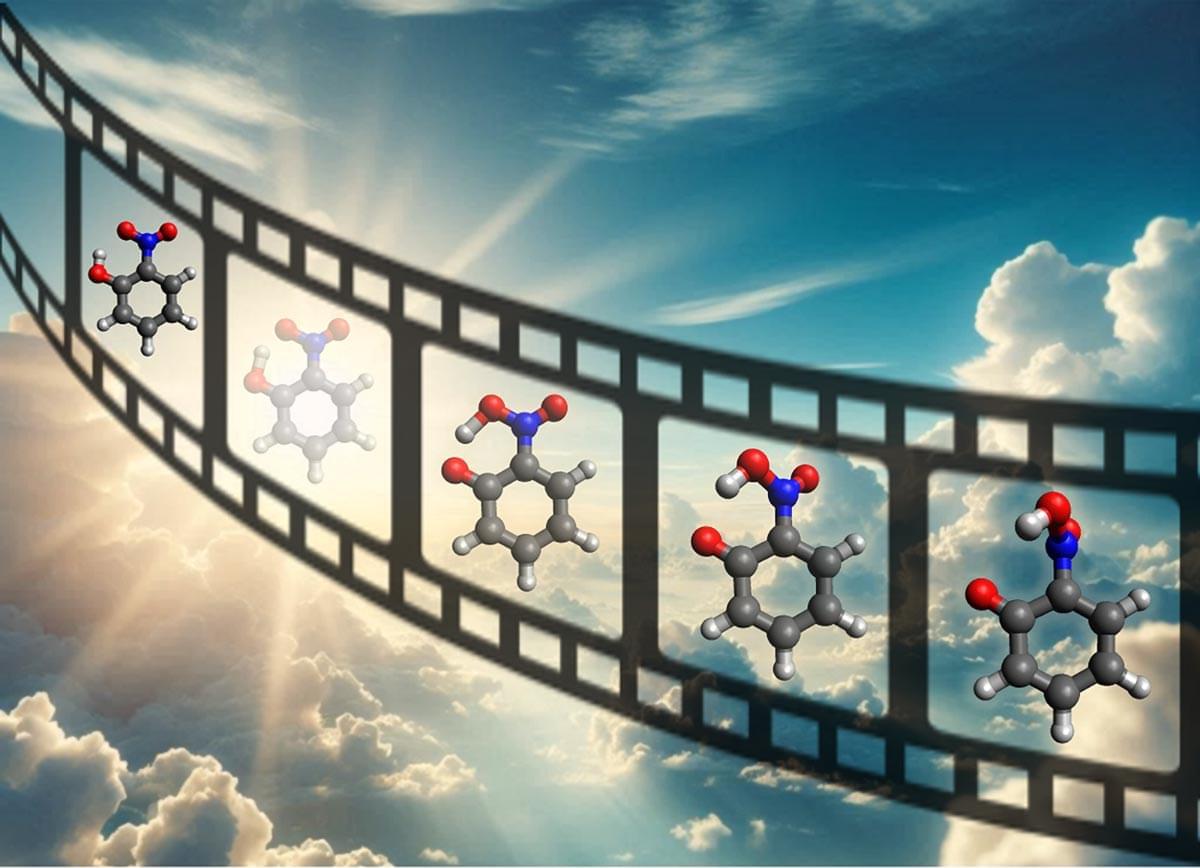
The interactions between light and nitroaromatic hydrocarbon molecules have important implications for chemical processes in our atmosphere that can lead to smog and pollution. However, changes in molecular geometry due to interactions with light can be very difficult to measure because they occur at sub-Angstrom length scales (less than a tenth of a billionth of a meter) and femtosecond time scales (one millionth of a billionth of a second).
The relativistic ultrafast electron diffraction (UED) instrument at the Linac Coherent Light Source (LCLS) at SLAC National Accelerator Laboratory provides the necessary spatial and time resolution to observe these ultrasmall and ultrafast motions. The LCLS is a Department of Energy (DOE) Office of Science light source user facility.
In this research, scientists used UED to observe the relaxation of photoexcited o–nitrophenol. Then, they used a genetic structure fitting algorithm to extract new information about small changes in the molecular shape from the UED data that were imperceptible in previous studies. Specifically, the experiment resolved the key processes in the relaxation of o-nitrophenol: proton transfer and deplanarization (i.e., a rotation of part of the molecule out of the molecular plane). Ab-initio multiple spawning simulations confirmed the experimental findings. The results provide new insights into proton transfer-mediated relaxation and pave the way for studies of proton transfer in more complex systems.

Israeli food-tech startup Finally Foods has developed the world’s first genetically engineered potatoes containing cow-milk protein, a breakthrough that could revolutionize dairy production.
The company, part of Strauss Group’s The Kitchen food-tech incubator, is set to launch its first field trial next month in southern Israel, where the modified potatoes will be cultivated.
Once harvested, the potatoes will be processed to extract casein protein powder, a key component in dairy production. Casein, which makes up 80% of milk proteins, is essential for cheese-making and provides melting, stretching and foaming properties in dairy products.
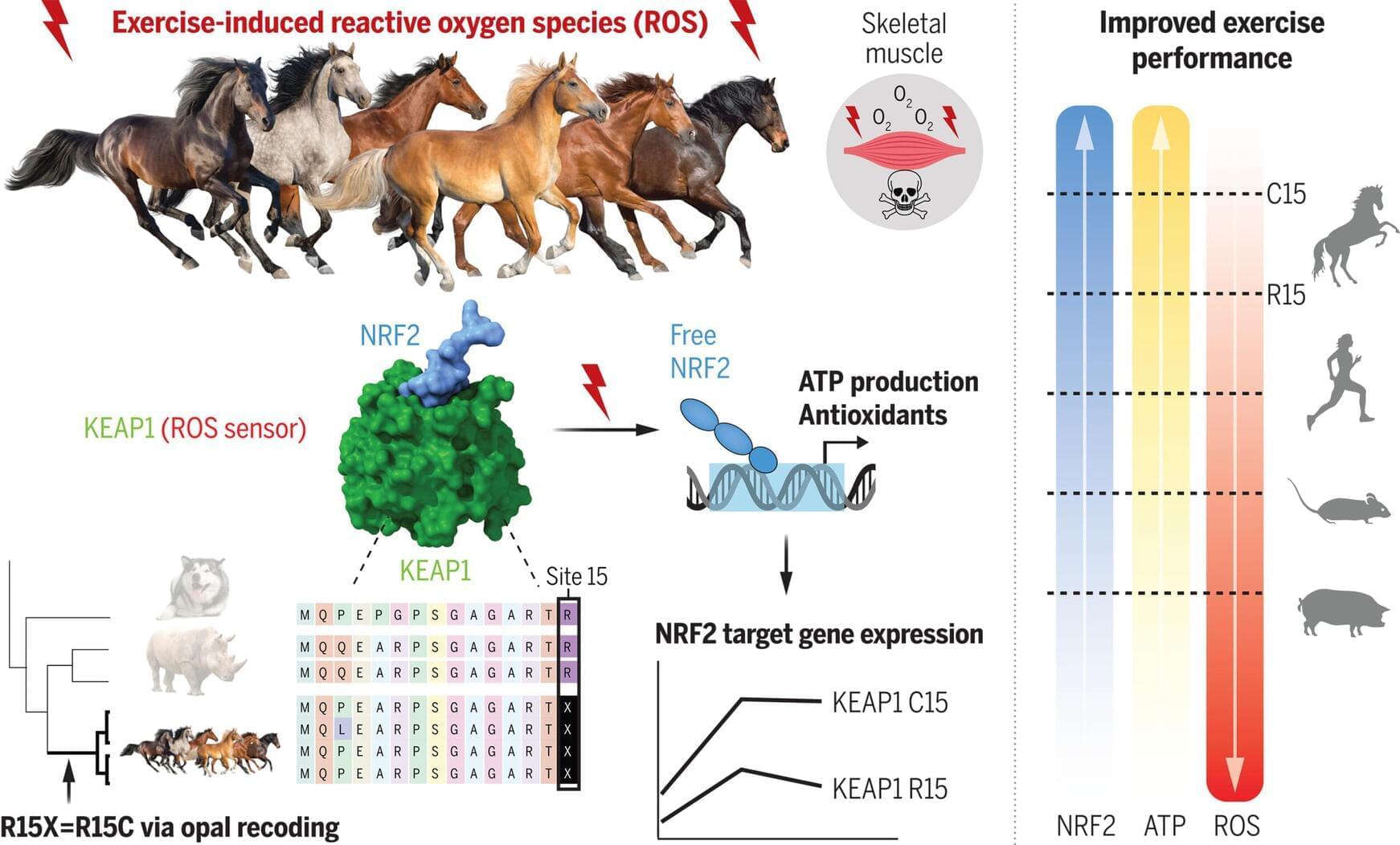
A genetic mutation in horses that would typically halt protein production has become a molecular asset. Researchers at Johns Hopkins University and Vanderbilt University have identified a rare instance of genetic recoding that enhances oxygen metabolism and energy production in horses, donkeys, and zebras.
The findings, published in Science, provide insight into the genetic foundation of exceptional equine athletic ability, and hint at an entirely new way of dealing with stop codons.
Few mammals match horses in aerobic performance. Muscle tissue in thoroughbreds consumes oxygen at rates exceeding 360 liters per minute. Oxygen uptake per unit of body mass is more than twice that of elite human athletes. While many genes involved in muscle structure and locomotion have been studied, the genetic basis for this level of metabolic output has remained unclear.
🚀 Imagine a future where living to 150 is possible—but only if you give up sugar, take ice-cold showers, and inject custom-engineered bacteria. Would you do it? In this deep dive into longevity science, we uncover the shocking truth about genetics, lifestyle, biohacking, and whether living longer actually makes life better. Plus, the real reason happiness might be the ultimate anti-aging hack! 🤯💡
#Longevity #AntiAging #Biohacking #LiveLonger #Science #Health #Wellness #LongevitySecrets #HealthyAging #LifeExtension
As our bodies grow, cells proliferate to form tissues, and cells frequently have to be repaired or replaced throughout life. But the genome can also become less stable over time, or may pick up mutations that can lead to disease; these and other processes can cause cells to enter a state in which they stop dividing, known as senescence. Senescent cells become more common as we age. There also tends to be more inflammation as we age, but the link between increasing instability in the genome and inflammation is not well understood. Now scientists have reported a direct connection between DNA instability and inflammation in senescent cells. The findings have been reported in Nature Communications.
“In addition to no longer growing and proliferating, the other hallmark of senescent cells is that they have this inflammatory program causing them to secrete inflammatory molecules,” noted senior study author Peter Adams, Ph.D., director and professor of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys.

The very public sharing of Gene Hackman and Betsy Arakawa’s health details raises ethical questions over privacy.
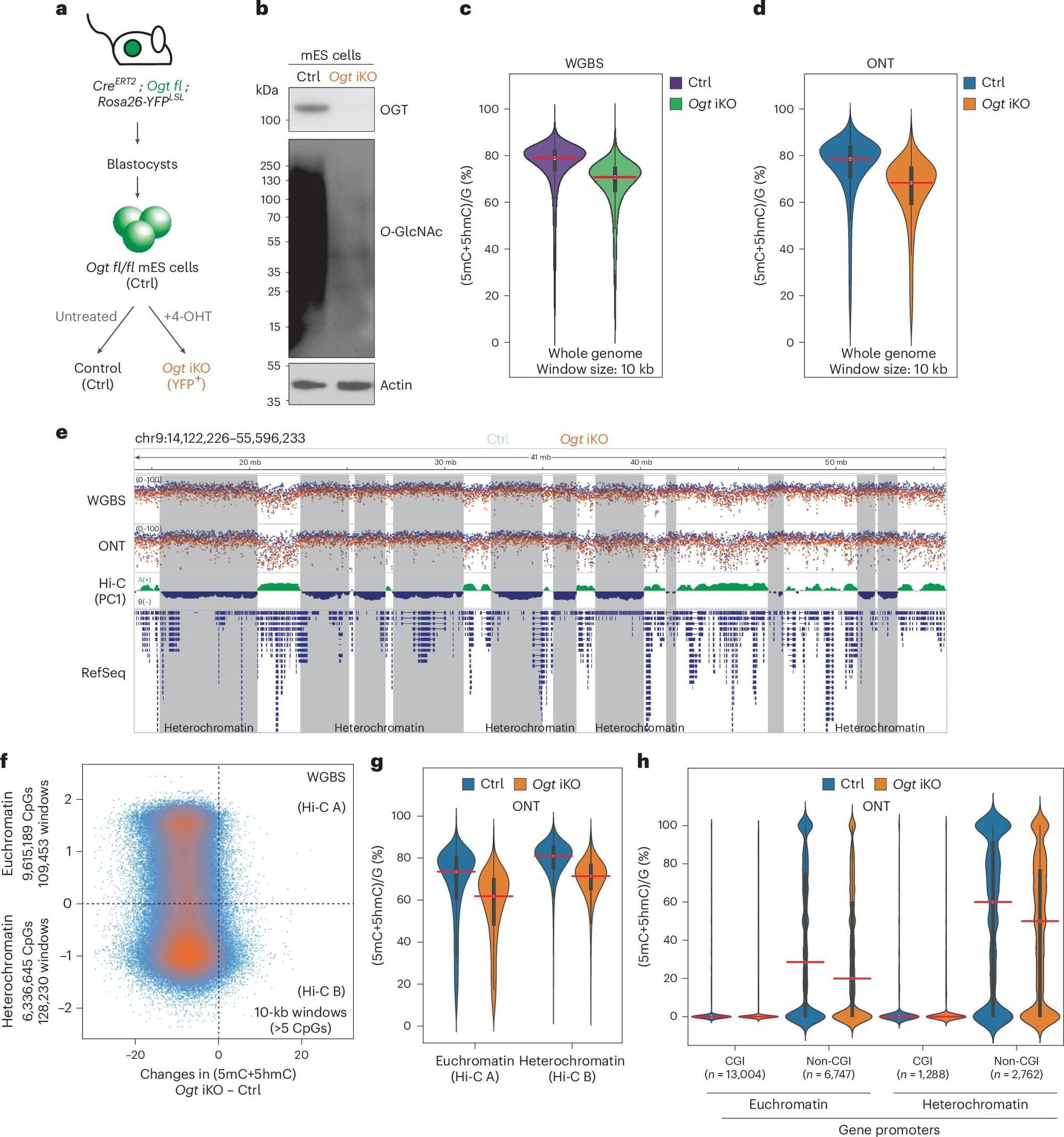
You may have heard of the fantastic-sounding “dark side of the genome.” This poorly studied fraction of DNA, known as heterochromatin, makes up around half of your genetic material, and scientists are now starting to unravel its role in your cells.
For more than 50 years, scientists have puzzled over the genetic material contained in this “dark DNA.” But there’s a growing body of evidence showing that its proper functioning is critical for maintaining cells in a healthy state. Heterochromatin contains tens of thousands of units of dangerous DNA, known as “transposable elements” (or TEs). TEs remain silently “buried” in heterochromatin in normal cells—but under many pathological conditions they can “wake up” and occasionally even “jump” into our regular genetic code.
And if that change benefits a cell? How wonderful! Transposable elements have been co-opted for new purposes through evolutionary history—for instance the RAG genes in immune cells and the genes required for driving the development of the placenta and mammalian evolution have been derived from TEs.
