In humans and other multicellular organisms, cells multiply. This defining feature allows embryos to grow into adulthood, and enables the healing of the many bumps, bruises and scrapes along the way.
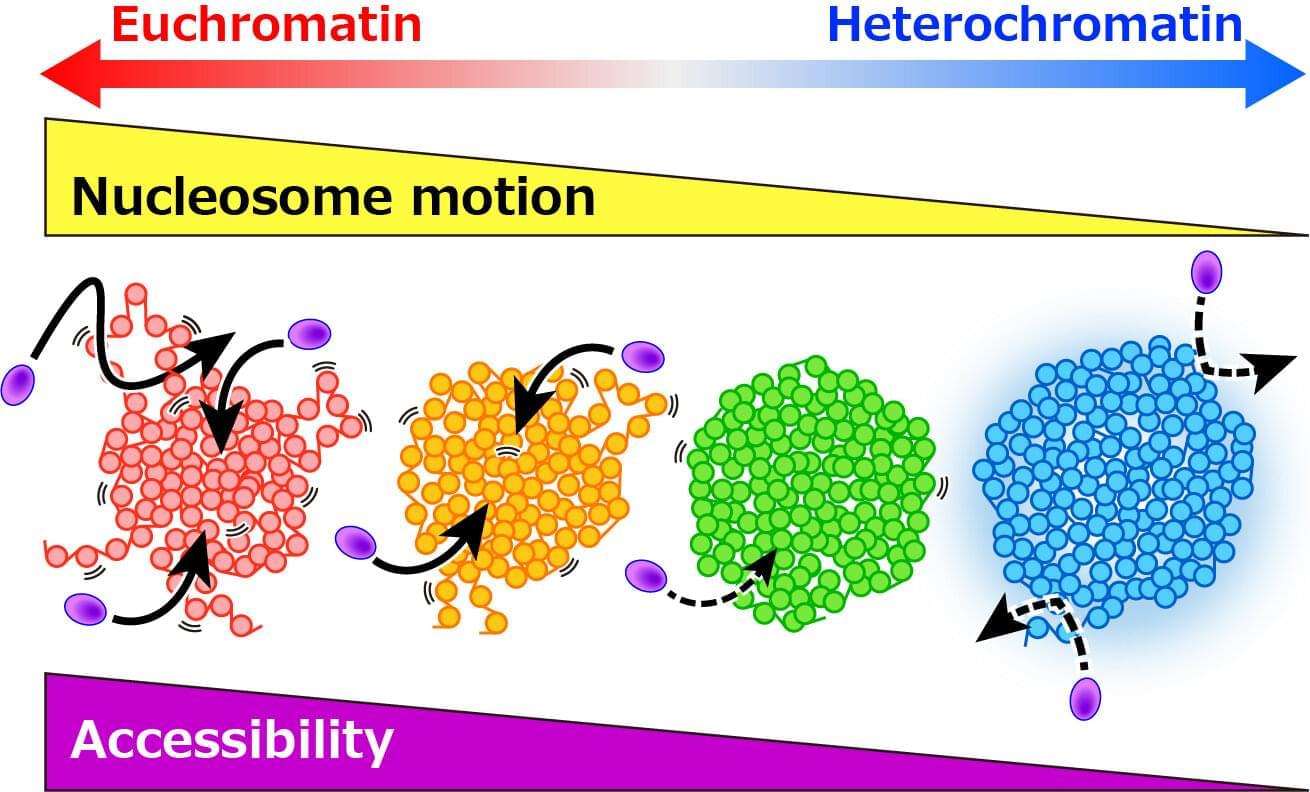
Certain factors can cause cells to abandon this characteristic and enter a zombie-like state known as senescence where they persist but no longer divide to make new cells. Our bodies can remove these senescent cells that tend to pile up as we age. The older we get, however, the less efficient our immune systems become at doing so.
“In addition to no longer growing and proliferating, the other hallmark of senescent cells is that they have this inflammatory program causing them to secrete inflammatory molecules,” said Peter Adams, Ph.D., director and professor of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and senior and co-corresponding author of the study.