“These neurons are playing an outsized role in hyperglycemia and type 2 diabetes,” said UW Medicine endocrinologist Dr. Michael Schwartz, corresponding author of the paper.
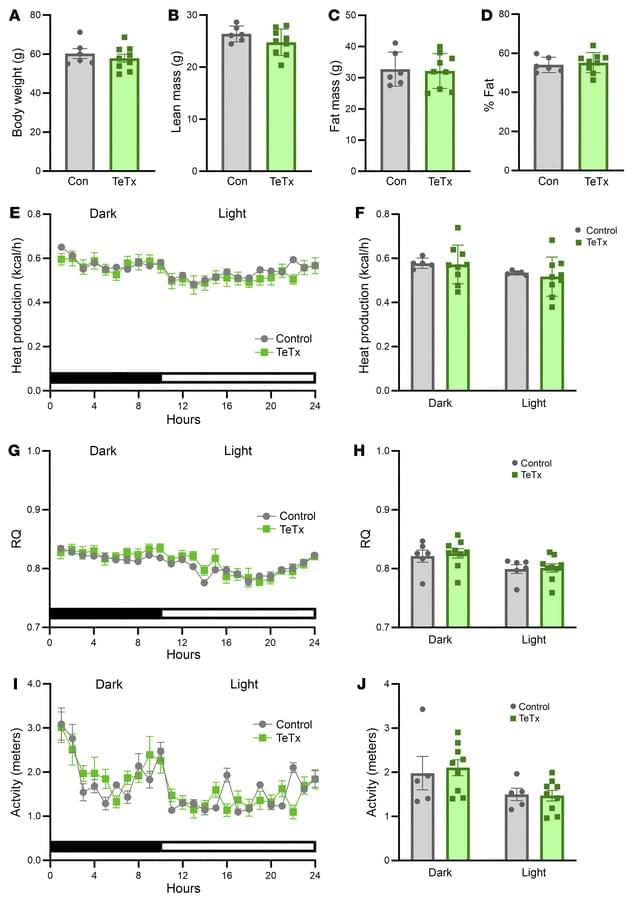
To determine if these neurons contribute to elevated blood sugar in diabetic mice, researchers employed a widely used viral genetics approach to make AgRP neurons express tetanus toxin, which prevents the neurons from communicating with other neurons.
Unexpectedly, this intervention normalized high blood sugar for months, despite having no effect on body weight or food consumption.
Conventional wisdom is that diabetes, particularly type 2 diabetes, stems from a combination of genetic predisposition and lifestyle factors, including obesity, lack of physical activity and poor diet. This mix of factors leads to insulin resistance or insufficient insulin production.
Until now, scientists have traditionally thought the brain doesn’t play a role in type 2 diabetes, according to Schwartz.
The paper challenges this and is a “departure from the conventional wisdom of what causes diabetes,” he said.
The new findings align with studies published by the same scientists showing that injection of a peptide called FGF1 directly into the brain also causes diabetes remission in mice. This effect was subsequently shown to involve sustained inhibition of AgRP neurons.