George Church’s lab at Harvard Medical School is working to make humans immune to all viruses, eliminate genetic diseases and reverse the aging process. Scott Pelley reports on how close the geneticist’s team is to a breakthrough.


The quest to live longer and healthier is not new. But the concept of reversing aging has recently stunned both the scienftific community and the public in general. Scientists have been able to reverse aging by 2.5 years to some participants in a groundbreaking experiment in the field of age reversal.
World leading scientists in the field of aging like David Sinclair think that aging is the ultimate disease that needs a cure. If scientsits were able to shed 2.5 years to the participants genomic age, the question raises itself, are we going to see an age reversal of a decade or more in the coming years?
#reverseaging #science #sciencetime
SUBSCRIBE to our channel “Science Time” and ring the bell to never miss out videos like this: https://www.youtube.com/sciencetime24
Source: Fahy, G. M. et al. Aging Cell https://doi.org/10.1111/acel.13028 (2019)
Similar videos:

Last week, a woman named Victoria Gray became the first person in the U.S. to have her cells edited with CRISPR. The 41-year-old patient was suffering from sickle cell anemia.
RELATED: FIRST HUMAN TRIAL USING CRISPR GENE-EDITING IN US BEGINS
The condition, caused by a genetic mutation that messes with the shape of red blood cells, causes havoc on patients, and to make things even worse, the options for treatment are very limited and ineffective. The only current treatment for sickle cell anemia patients is a donor transplant that works for just 10% of patients, but all that is about to change.
New post!
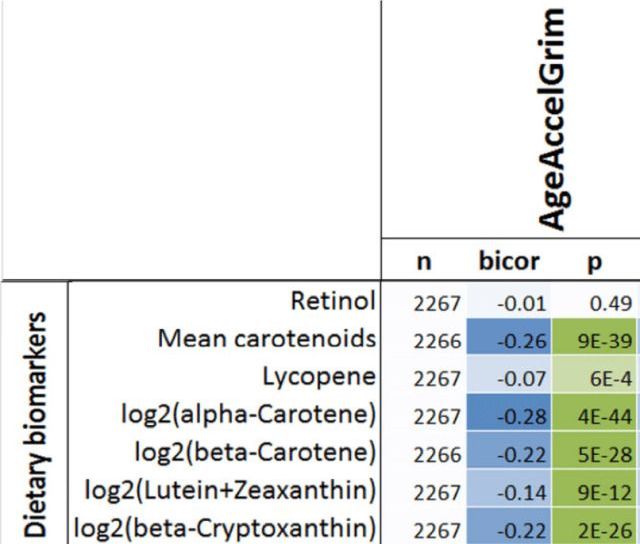
Having a faster rate of epigenetic aging, as measured by the epigenetic age metric, AgeAccelGrim, is associated with a significantly increased risk of death for all causes in a variety of cohorts, including the Framingham Heart Study (FHS), the Women’s Health Initiative (WHI) study, the InChianti study, the Jackson Heart Study (JHS), and collectively, when evaluated as a meta-analysis (Lu et al. 2019):
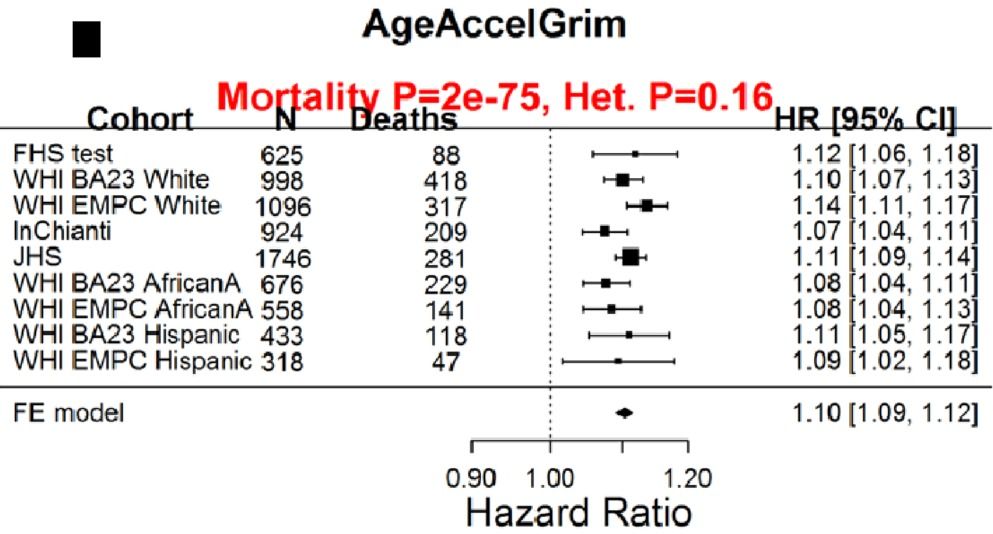
With the goal of minimizing disease risk and maximizing longevity, can epigenetic aging be slowed? Shown below is the correlation between dietary components with AgeAccelGrim. Dietary factors that were significantly associated (the column labelled, “p”) with a younger epigenetic age were carbohydrate intake, dairy, whole grains, fruit, and vegetables. In contrast, dietary fat intake and red meat were associated with older epigenetic ages (Lu et al. 2019):

Amidst rising hopes for using CRISPR gene editing tools to repair deadly mutations linked to conditions like cystic fibrosis and sickle cell disease, a study in Communications Biology describes a new innovation that could accelerate this work by rapidly revealing unintended and potentially harmful changes introduced by a gene editing process.
“We’ve developed a new process for rapidly screening all of the edits made by CRISPR, and it shows there may be many more unintended changes to DNA around the site of a CRISPR repair than previously thought,” said Eric Kmiec, Ph.D., director of ChristianaCare’s Gene Editing Institute and the principle author of the study.
The study describes a new tool developed at the Gene Editing Institute that in just 48 hours can identify “multiple outcomes of CRISPR-directed gene editing,” a process that typically required up to two months of costly and complicated DNA analysis.
Awesome!
A new approach to programing cancer-fighting immune cells called CAR-T cells can prolong their activity and increase their effectiveness against human cancer cells grown in the laboratory and in mice, according to a study by researchers at the Stanford University School of Medicine.
The ability to circumvent the exhaustion that the genetically engineered cells often experience after their initial burst of activity could lead to the development of a new generation of CAR-T cells that may be effective even against solid cancers—a goal that has until now eluded researchers.
The studies were conducted in mice harboring human leukemia and bone cancer cells. The researchers hope to begin clinical trials in people with leukemia within the next 18 months and to eventually extend the trials to include solid cancers.

Biological weapons could be built which target individuals in a specific ethnic group based on their DNA, a report by the University of Cambridge has warned.
Researchers from Cambridge’s Centre for the Study of Existential Risk (CSER) said the government was failing to prepare for ‘human-driven catastrophic risks’ that could lead to mass harm and societal collapse.
In recent years advances in science such as genetic engineering, and artificial intelligence (AI) and autonomous vehicles have opened the door to a host of new threats.
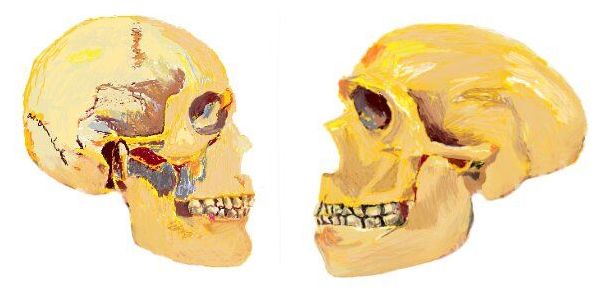
A new University of Barcelona study reveals the first empirical genetic evidence of human self-domestication, a hypothesis that humans have evolved to be friendlier and more cooperative by selecting their companions depending on their behaviour. Researchers identified a genetic network involved in the unique evolutionary trajectory of the modern human face and prosociality, which is absent in the Neanderthal genome. The experiment is based on Williams Syndrome cells, a rare disease.
The study, published in Science Advances, results from the collaboration between a UB team led by Cedric Boeckx, ICREA professor from the Section of General Linguistics at the Department of Catalan Philology and General Linguistics, and member of the Institute of Complex Systems of the UB (UBICS), and researchers from the team led by Giuseppe Testa, lecturer at the University of Milan and the European Institute of Oncology.
