This video explains in detail about the post transcriptional modifications on histone proteins, epigenetics, methylation, phosphorylation, acetylation, ubiquitylation and sumoylation.
Category: genetics – Page 421
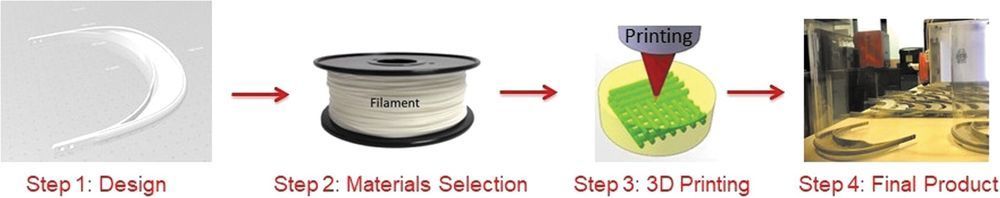
Additive Manufacturing Can Assist in the Fight Against COVID-19 and Other Pandemics and Impact on the Global Supply Chain
The high demand on medical devices and personal protective equipment (PPE) during the COVID-19 crisis left millions of health care professionals unprotected in the middle of this situation, as governments around the world were not prepared for such pandemic. The three-dimensional printing (3DP) community, from universities to 3DP enthusiasts with printers at home, was there to support hospitals from day 1 on this demand by providing PPE and other medical supplies (e.g., face shields and valves for respiratory machines). This editorial covers the importance of 3DP in the fight against COVID-19 and how this can be used to tackle potential pandemics and support the supply chain.
After a series of cases of pneumonia in Wuhan, the capital city of Hubei province (China), the Chinese health authorities announced in January 2020 that a novel coronavirus, officially known as severe acute respiratory syndrome coronavirus (SARS-CoV)-2, was responsible for these cases.1 SARS-CoV-2, the virus that causes the coronavirus disease (COVID-19), was not detected before the recent pandemic and has been known to be genetically similar to SARS-CoV.1 The COVID-19 is transmitted mainly through contact with an infected individual, through droplets that are produced when the patient coughs or sneezes or through droplets from the saliva or nasal cavity.1,2 To avoid transmission, it is very important to implement individual hygiene measures and especially the use of personal protective equipment (PPE). However, the lack of PPE and other key resources during the COVID-19 crisis has been a constant problem, leaving many health care professionals across the world unprotected.
Dealing with a pandemic, such as COVID-19, is an unprecedented situation in this modern globalized word, which has created extraordinary emergency that is particularly affecting the supply chain.3 The supply chain disruptions, in combination with the enormous needs for medical devices and protective health care material, have created the need of new initiatives and the use of emerging technologies such as three-dimensional printing (3DP) to come forward and support the health care professionals and supply chain.
To understand the machinery of life, this scientist breaks it on purpose
“We expected that the hammer of natural selection also comes down randomly, but that is not what we found,” he said. “Rather, it does not act randomly but has a strong bias, favoring those mutations that provide the largest fitness advantage while it smashes down other less beneficial mutations, even though they also provide a benefit to the organism.”
In other words, evolution is not a multitasker when it comes to fixing problems.
“It seems that evolution is myopic,” Venkataram said. “It focuses on the most immediate problem, puts a Band-Aid on and then it moves on to the next problem, without thoroughly finishing the problem it was working on before.”
“It turns out the cells do fix their problems but not in the way we might fix them,” Kaçar added. “In a way, it’s a bit like organizing a delivery truck as it drives down a bumpy road. You can stack and organize only so many boxes at a time before they inevitably get jumbled around. You never really get the chance to make any large, orderly arrangement.”
Why natural selection acts in this way remains to be studied, but what the research showed is that, overall, the process results in what the authors call “evolutionary stalling”—while evolution is busy fixing one problem, it does at the expense of all other issues that need fixing. They conclude that at least in rapidly evolving populations, such as bacteria, adaptation in some modules would stall despite the availability of beneficial mutations. This results in a situation in which organisms can never reach a fully optimized state.
“The system has to be capable of being less than optimal so that evolution has something to act on in the face of disturbance—in other words, there needs to be room for improvement,” Kaçar said.
Kaçar believes this feature of evolution may be a signature of any self-organizing system, and she suspects that this principle has counterparts at all levels of biological hierarchy, going back to life’s beginnings, possibly even to prebiotic times when life had not yet materialized.
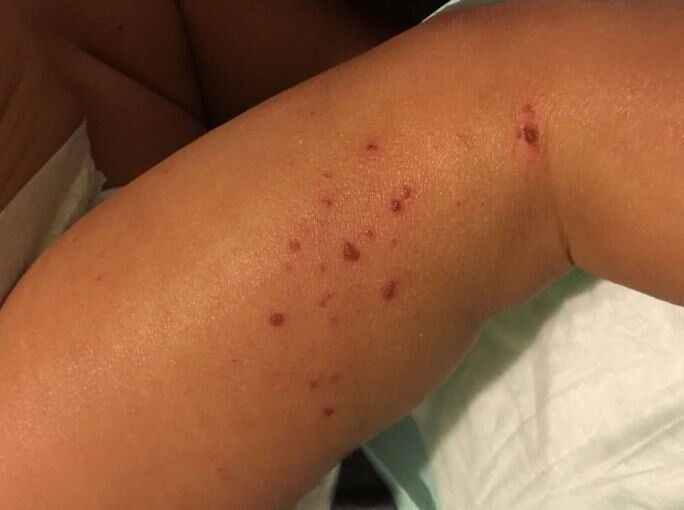
Scientists demonstrate how genetic variations cause eczema
New research supported by the National Institutes of Health delineates how two relatively common variations in a gene called KIF3A are responsible for an impaired skin barrier that allows increased water loss from the skin, promoting the development of atopic dermatitis, commonly known as eczema. This finding could lead to genetic tests that empower parents and physicians to take steps to potentially protect vulnerable infants from developing atopic dermatitis and additional allergic diseases.
Atopic dermatitis is an inflammatory skin condition that affects up to 20% of children in developed countries. This chronic disease is characterized by dry, thickened and intensely itchy skin, particularly in skin folds. People with eczema are more susceptible to bacterial, viral and fungal skin infections and frequently develop additional allergic diseases such as asthma.
KIF3A is a gene that codes for a protein involved in generating signals from the outside to the inside of a cell, part of a complex sensory apparatus. Previously, scientists had identified an association between two genetic variations in KIF3A and asthma in children who also had eczema. In the new study, the researchers found that these variations, or single nucleotide polymorphisms (SNPs), changed parts of the KIF3A gene to a form that can regulate, through a process called methylation, the rate at which a gene is transcribed into the blueprint for protein production. The investigators confirmed that skin and nasal-lining cells from people with the KIF3A SNP variants had more methylation and contained fewer blueprints for the KIF3A protein than cells in which KIF3A lacked the SNPs. In addition, the researchers demonstrated that people with the SNP-created regulating sites had higher levels of water loss from the skin.

FDA Approves Viltolarsen for Duchenne Muscular Dystrophy
The US Food and Drug Administration (FDA) has approved viltolarsen (Viltepso; NS Pharma) for the treatment of patients with Duchenne muscular dystrophy amenable to exon 53 skipping, making it only the second FDA-approved therapy for this specific DMD gene mutation.
The agent from NS Pharma, delivered via weekly intravenous infusion, was granted accelerated approval via its priority review, fast track, orphan drug, and rare disease designations after its new drug application was accepted earlier this year. In March, NS Pharma launched an expanded access program for qualified patients.
The approval was granted based on findings from a phase 2 clinical trial (NCT02740972) and long-term extension study, details of which were recently published in JAMA Neurology. Among 16 participants age 4 to 9, significant drug-induced dystrophin production was observed in both viltolarsen dose cohorts (40 mg/kg per week: mean, 5.7% [range, 3.2–10.3] of normal; 80 mg/kg per week: mean, 5.9% [range, 1.1–14.4] of normal), with 15 (94%) patients achieving dystrophin levels greater than 2% of normal and 14 of 16 (88%) achieving levels greater than 3% of normal.

Genetics in Microscopic Marine Life: The Plankton Potential
While satellite imaging lets researchers observe the outer life of plankton populations, the complex genetics in microscopic marine life have made looking inward more challenging. According to a new study published in Nature Methods, researchers from the University of East Anglia were able to deliver and express foreign DNA in 13 species that have never before transformed. They were also able to evaluate the potential cause of non-transformation in 17 other species; in turn, laying the foundation for an expanded understanding of genomes discovered in plankton.
The sheer variety of plankton potential — from antibacterial compounds to antiviral and antifungal solutions — makes this a worthwhile endeavor. If scientists can create reliable methods to modify phytoplankton, it should be possible to reduce their toxic impact, better control their bloom cycle and even increase the photosynthetic output — all critical in the fight to keep our oceans blue and our terra firma green.
As noted by Science Magainze, the international research team used a variety of methods to modify plankton DNA. For some species, shooting tiny gold or tungsten particles covered with DNA through cell walls produced the best result. For others, jolts of electricity made cell walls “leaky” and allowed new DNA to seep through. Specific protist successes included modification of a fish-killing toxic plankton species, and one that infects both mollusks and amphibians. While these discoveries don’t present a complete understanding of the genetics in microscopic marine life, they provide a key testing protocol: By modifying genetic structure and then observing how plankton react, teams could uncover ways to boost antibiotic resistance or lower infectious impact. According to lead UK study author Thomas Mock, “These insights will improve our understanding about their role in the oceans, and they are invaluable for biotechnological applications such as building factories for biofuel or the production of bioactive compounds.”
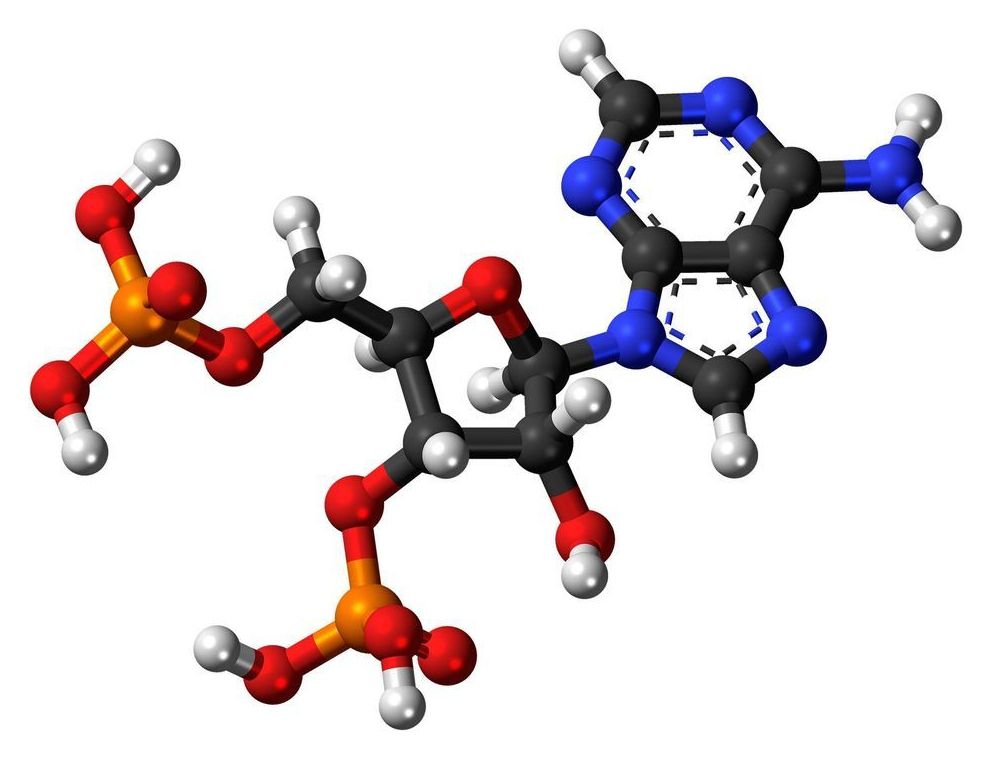
A cancer mystery more than 40 years old is solved thanks to epigenetics
Before the first oncogene mutations were discovered in human cancer in the early 1980s, the 1970s provided the first data suggesting alterations in the genetic material of tumors. In this context, the prestigious journal Nature published in 1975 the existence of a specific alteration in the transformed cell: an RNA responsible for carrying an amino acid to build proteins (transfer RNA) was missing a piece, the enigmatic nucleotide ‘Y.’
After that outstanding observation, virtually no developments were made for forty-five years on the causes and consequences of not having the correct base in RNA.
In an article published in Proceedings of the National Academy of Sciences (PNAS) by the group of Dr. Manel Esteller, Director of the Josep Carreras Leukaemia Research Institute, ICREA Research Professor and Professor of Genetics at the University of Barcelona has solved this mystery by observing that in cancer cells the protein that generates the nucleotide Y is epigenetically inactivated, causing small but highly aggressive tumors.

Is There A Way To Reverse Age
Harvard geneticist Dr. George Church is “turning on” youth-promoting genes. In this exclusive interview Dr. Church explains how he expects to achieve human age reversal in the foreseeable future.
Scientifically reviewed by: Dr. Amanda Martin, DC, on August 2020. Written By Dr. Shelly Xuelai Fan.

Thymus Regeneration, Immunorestoration, and Insulin Mitigation Extension Trial
Last year information was released concerning rejuvenation of the thymus which resulted in a reversal of the epigenetic clock an average of 2.5 years in a small trial of 9 people costing $10,000 per person. You can get this done too. A company has formed called Intervene Immune which will take on volunteers for the process. It is not funded so you would have to pay out pf pocket though eventually the cost may come down and they can provide financing. You do not have to travel to California to get this done. Cost prohibits me, and I may or may not be eligible as I have IBS though that is not on the exclusion list. I emailed them concerning all this which is how I got the information.
https://www.surveymonkey.com/r/TRIIMX
The TRIIM-X trial is an expanded pilot clinical study that will evaluate a personalized combination treatment regimen for thymus regeneration. The thymus is a part of the immune system that declines markedly with age, and regenerating it may prevent or reverse key aspects of immunosenescence (immune system aging) and potentially prevent or reverse key parts of the aging process more generally. The study will evaluate biomarkers for epigenetic aging and immunosenescence, as well as evaluate established clinical measures and risk factors for prevention of physical frailty, cancer, cardiovascular disease, diabetes, dementia, and also infectious diseases, including flu and COVID-19.
The study uses multiple agents in combination with personalized doses of recombinant human growth hormone (somatropin), metformin, and DHEA, in a similar manner to how the combination treatment was applied in the earlier TRIIM trial at Stanford, which demonstrated strong statistical significance for the primary efficacy endpoints that will be evaluated in TRIIM-X. Somatropin is approved by the FDA for adult growth hormone deficiency and its use in the study is guided by prior safety data established for that use and also based on safety data available on its prior use in the TRIIM trial and in clinical practice in healthy elderly individuals. There will also be control groups that enable testing of biomarker variability and the contribution of individual medications within the combination treatment.
The objective of the study is to obtain information needed for designing an effective personalized and adaptive treatment regimen for a larger and more diverse study population, and to obtain additional proof of principle for the new use of the medications and biomarkers for preventive medicine. The duration of treatment in the TRIIM-X trial will be 12 months.
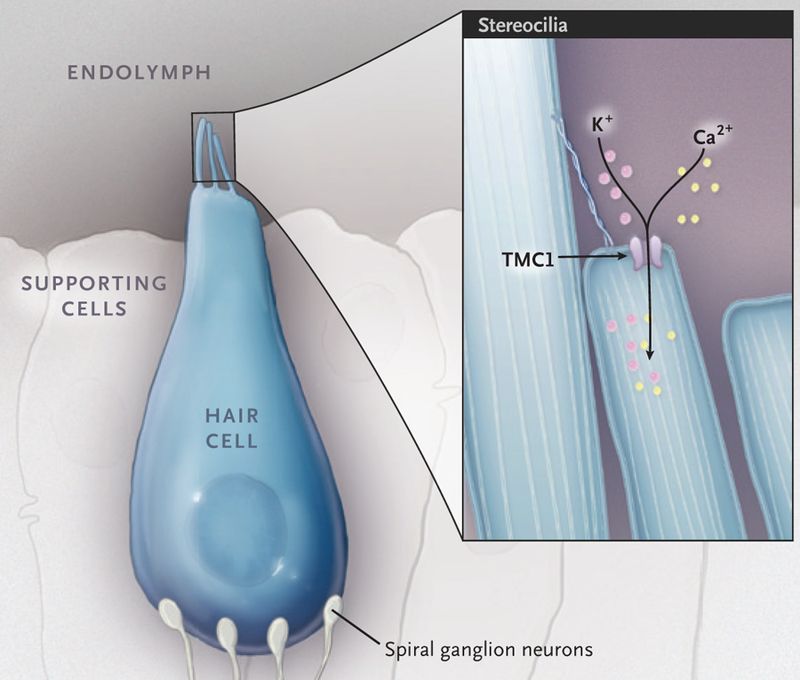
A CRISPR Way to Restore Hearing
Hereditary hearing loss is one of the most common disabilities among newborns, affecting approximately 1 in 1000 live-born babies. Most forms of hereditary hearing loss are nonsyndromic; 80% of affected newborns have hearing loss that is inherited in an autosomal recessive pattern, and in the remaining 20%, inheritance shows a dominant pattern.1
Many forms of hereditary hearing loss are caused by mutations in genes that affect the formation and function of cochlear hair cells — highly specialized sensory cells that play an important role in the detection and processing of sound.1 The hair cell has bundles of hair-like projections, called stereocilia, on its apical surface ( Fig. 1 ). The deflection of these bundles by sound results in the opening of mechanotransduction ion channels, which are located at the tips of the stereocilia, and consequently, in the depolarization of the hair-cell membrane. Mutations that affect the protein transmembrane channel-like 1 (TMC1), an integral component of the mechanotransduction complex, cause autosomal dominant and autosomal recessive forms of hearing loss.2 Correction of the dominant form of hearing loss in a mouse model of Tmc1 (termed “Beethoven”) was recently reported by Gao and colleagues.3