GMO 🦟
More than 20 million genetically modified mosquitoes are coming to the Florida Keys this year, in a landmark project by British biotech company Oxitec and Monroe County’s Mosquito Control District.

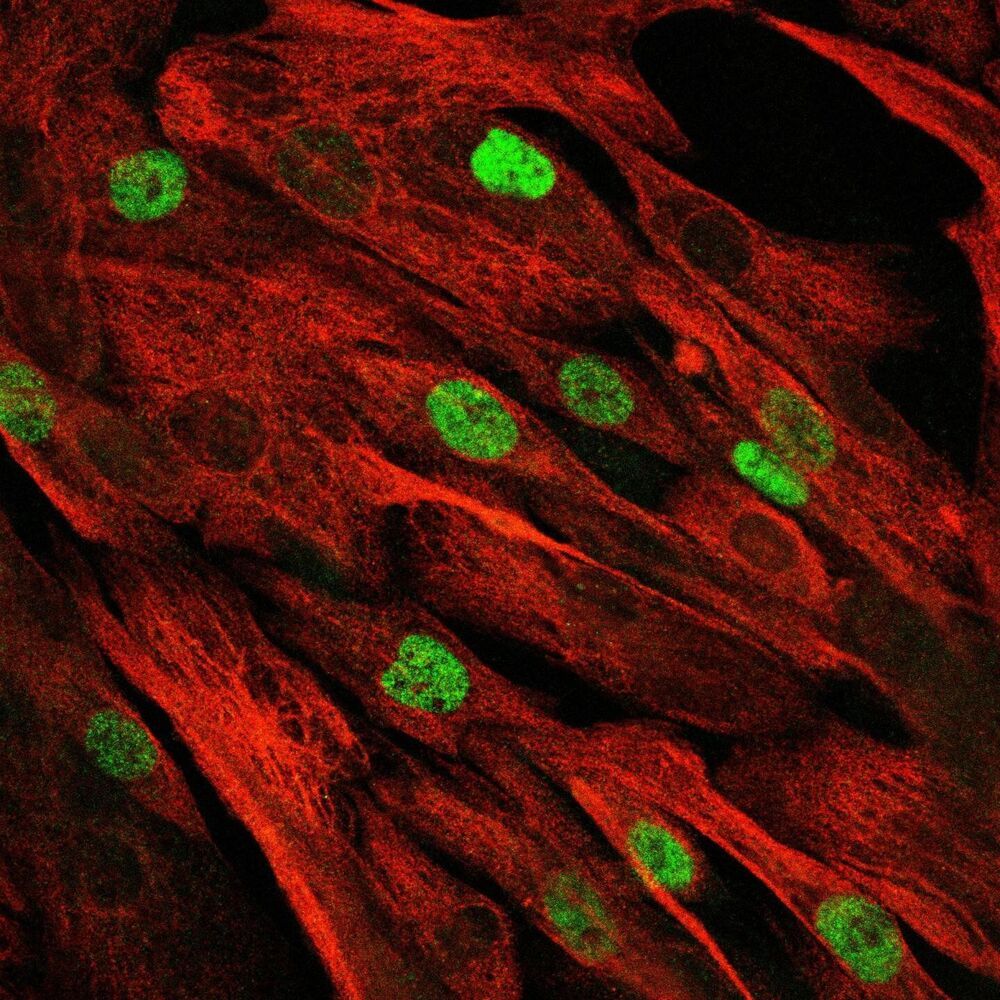
Muscle stem cells enable our muscle to build up and regenerate over a lifetime through exercise. But if certain muscle genes are mutated, the opposite occurs. In patients suffering from muscular dystrophy, the skeletal muscle already starts to weaken in childhood. Suddenly, these children are no longer able to run, play the piano or climb the stairs, and often they are dependent on a wheelchair by the age of 15. Currently, no therapy for this condition exists.
“Now, we are able to access these patients’ gene mutations using CRISPR-Cas9 technology,” explains Professor Simone Spuler, head of the Myology Lab at the Experimental and Clinical Research Center (ECRC), a joint institution of the Max Delbrück Center for Molecular Medicine in the Helmholtz Association and Charité — Universitätsmedizin Berlin. “We care for more than 2000 patients at the Charité outpatient clinic for muscle disorders, and quickly recognized the potential of the new technology.” The researchers immediately started working with some of the affected families, and have now presented their results in the journal JCI Insight. In the families studied, the parents were healthy and had no idea they possessed a mutated gene. The children all inherited a copy of the disease mutation from both parents.

For the first time, scientists have succeeded in extracting and analyzing Neandertal chromosomal DNA preserved in cave sediments.
The field of ancient DNA has revealed important aspects of our evolutionary past, including our relationships with our distant cousins, Denisovans, and Neandertals. These studies have relied on DNA from bones and teeth, which store DNA and protect it from the environment. But such skeletal remains are exceedingly rare, leaving large parts of human history inaccessible to genetic analysis.
To fill these gaps, researchers at the Max Planck Institute for Evolutionary Anthropology developed new methods for enriching and analyzing human nuclear DNA from sediments, which are abundant at almost every archaeological site. Until now, only mitochondrial DNA has been recovered from archaeological sediments, but this is of limited value for studying population relationships. The advent of nuclear DNA analyses of sediments provides new opportunities to investigate the deep human past.
Paper references for Levine’s Phenotypic Age calculator and aging.ai:
An epigenetic biomarker of aging for lifespan and healthspan:
https://pubmed.ncbi.nlm.nih.gov/29676998/
Population Specific Biomarkers of Human Aging: A Big Data Study Using South Korean, Canadian, and Eastern European Patient Populations:
https://pubmed.ncbi.nlm.nih.gov/29340580/

Harvard’s Wyss Institute has created a new gene-editing tool that enable scientist to perform millions of genetic experiments simultaneously.
Researchers from the Harvard’s Wyss Institute for Biologically Inspired Engineering have created a new gene-editing tool that can enable scientists to perform millions of genetic experiments simultaneously. They’re calling it the Retron Library Recombineering (RLR) technique, and it uses segments of bacterial DNA called retrons that can produce fragments of single-stranded DNA.
When it comes to gene editing, CRISPR-Cas9 is probably the most well-known technique these days. It’s been making waves in the science world in the past few years, giving researchers the tool they need to be able to easily alter DNA sequences. It’s more accurate than previously used techniques, and it has a wide variety of potential applications, including life-saving treatments for various illnesses.
However, the tool has some major limitations. It could be difficult to deliver CRISPR-Cas9 materials in large numbers, which remains a problem for studies and experiments, for one. Also, the way the technique works can be toxic to cells, because the Cas9 enzyme — the molecular “scissors” in charge of cutting strands of DNA — often cuts non-target sites as well.
Targeting a pathway that is essential for the survival of certain types of acute myeloid leukaemia could provide a new therapy avenue for patients, the latest research has found.
Researchers from the Wellcome Sanger Institute found that a specific genetic mutation, which is linked with poor prognosis in blood cancer, is involved in the development of the disease when combined with other mutations in mice and human cell lines.
The study, published today (30th April) in Nature Communications, provides a greater understanding of how the loss-of-function mutation in the CUX1 gene leads to the development and survival of acute myeloid leukaemia. The findings suggest that targeting a pathway that is essential for these cancer cells to continue growing could lead to new targeted therapies for some patients.
A bold project to read the complete genetic sequences of every known vertebrate species reaches its first milestone by publishing new methods and the first 25 high-quality genomes.
It’s one of the most audacious projects in biology today – reading the entire genome of every bird, mammal, lizard, fish, and all other creatures with backbones.
And now comes the first major payoff from the Vertebrate Genomes Project (VGP): near complete, high-quality genomes of 25 species, Howard Hughes Medical Institute (HHMI) Investigator Erich Jarvis with scores of coauthors report April 28, 2021, in the journal Nature. These species include the greater horseshoe bat, the Canada lynx, the platypus, and the kākāpō parrot – one of the first high-quality genomes of an endangered vertebrate species.
An acquired mutation in the cancer-causing gene PIK3CA can make blood vessel malformations in the brain worse, possibly explaining why these abnormal clusters sometimes rapidly increase in size and cause stroke or seizures, shows new research.
Research from the University of Pennsylvania and Duke University shows an acquired mutation in the cancer-causing gene PIK3CA can trigger uncontrolled growth in cerebral cavernous malformations often leading to strokes or seizures in those affected.
While the CRISPR-Cas9 gene editing system has become the poster child for innovation in synthetic biology, it has some major limitations. CRISPR-Cas9 can be programmed to find and cut specific pieces of DNA, but editing the DNA to create desired mutations requires tricking the cell into using a new piece of DNA to repair the break. This bait-and-switch can be complicated to orchestrate, and can even be toxic to cells because Cas9 often cuts unintended, off-target sites as well.
Alternative gene editing techniques called recombineering instead perform this bait-and-switch by introducing an alternate piece of DNA while a cell is replicating its genome, efficiently creating genetic mutations without breaking DNA. These methods are simple enough that they can be used in many cells at once to create complex pools of mutations for researchers to study. Figuring out what the effects of those mutations are, however, requires that each mutant be isolated, sequenced, and characterized: a time-consuming and impractical task.
Researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Harvard Medical School (HMS) have created a new gene editing tool called Retron Library Recombineering (RLR) that makes this task easier. RLR generates up to millions of mutations simultaneously, and “barcodes” mutant cells so that the entire pool can be screened at once, enabling massive amounts of data to be easily generated and analyzed. The achievement, which has been accomplished in bacterial cells, is described in a recent paper in PNAS.

Mitochondria are the energy suppliers of our body cells. These tiny cell components have their own genetic material, which triggers an inflammatory response when released into the interior of the cell. The reasons for the release are not yet known, but some cardiac and neurodegenerative diseases as well as the aging process are linked to the mitochondrial genome. Researchers at the Max Planck Institute for Biology of Aging and the CECAD Cluster of Excellence in Aging research have investigated the reasons for the release of mitochondrial genetic material and found a direct link to cellular metabolism: when the cell’s DNA building blocks are in short supply, mitochondria release their genetic material and trigger inflammation. The researchers hope to find new therapeutic approaches by influencing this metabolic pathway.
Our body needs energy—for every metabolic process, every movement and for breathing. This energy is produced in tiny components of our body cells, the so-called mitochondria. Unlike other cell components, mitochondria have their own genetic material, mitochondrial DNA. However, in certain situations, mitochondria release their DNA into the interior of the cell, causing a reaction from the cell’s own immune system and being associated with various diseases as well as the aging process. The reasons for the release of mitochondrial DNA are not yet known.