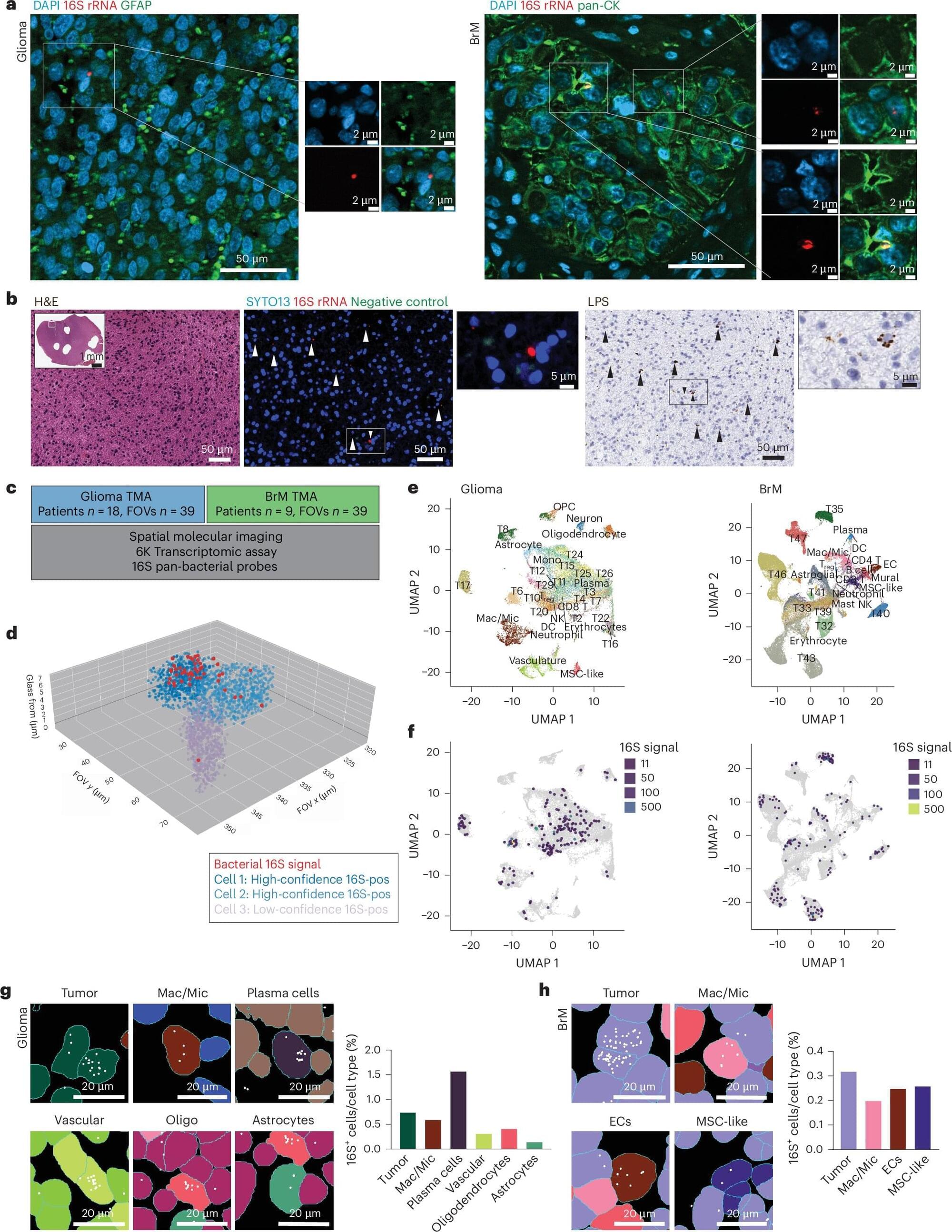
A new study is providing a clearer picture of the genetic landscape of major depression, revealing that the disorder may have fundamentally different biological roots depending on the age at which it first appears. The research, published in Nature Genetics, found that depression beginning in adolescence or young adulthood has a stronger genetic basis, is linked to early brain development, and carries a much higher genetic association with suicide attempts compared to depression that starts later in life.
Major depressive disorder is recognized as a clinically diverse condition, meaning its symptoms and course can vary substantially from person to person. Researchers have long suspected that this clinical variability might stem from different underlying causes.
One of the most apparent distinctions among individuals with depression is their age at onset. Depression that emerges early in life is often associated with more severe outcomes, including suicidal behavior, while late-onset depression has been linked more frequently to cognitive decline and cardiovascular problems.