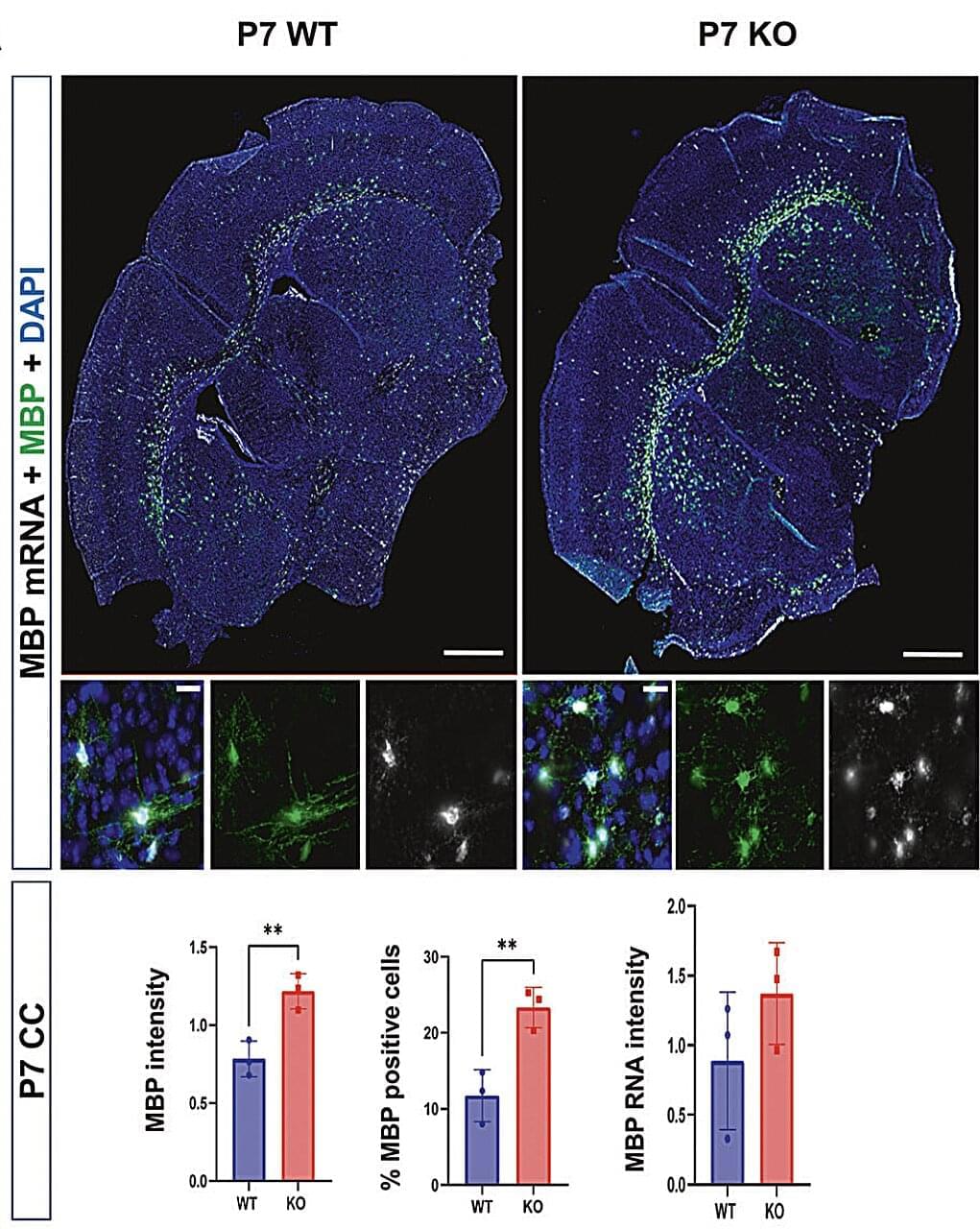
This ‘fundamental’ study uncovers how directing pyruvate into mitochondria can shrink cells by shifting their metabolism away from building amino acids and proteins.
This fundamental work demonstrates that compartmentalized cellular metabolism is a dominant input into cell size control in a variety of mammalian cell types and in Drosophila. The authors show that increased pyruvate import into the mitochondria in liver-like cells and in primary hepatocytes drives gluconeogenesis but reduces cellular amino acid production, suppressing protein synthesis. The evidence supporting the conclusions is compelling, with a variety of genetic and pharmacologic assays rigorously testing each step of the proposed mechanism. This work will be of interest to cell biologists, physiologists, and researchers interested in cell metabolism, and is significant because stem cells and many cancers exhibit metabolic rewiring of pyruvate metabolism.