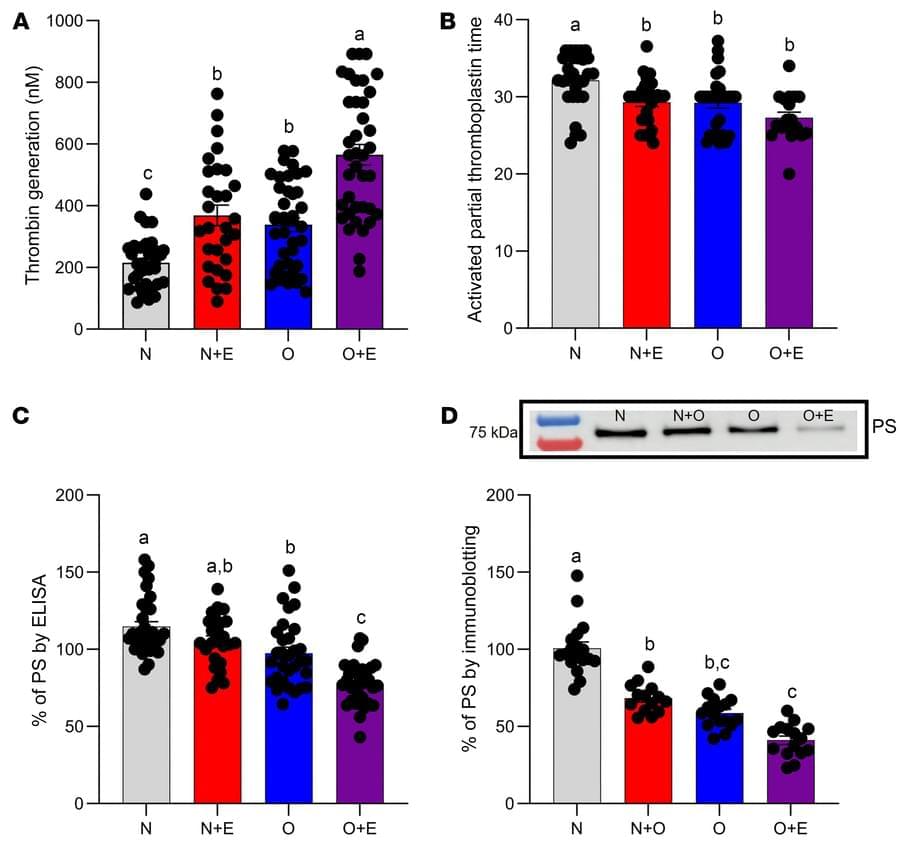
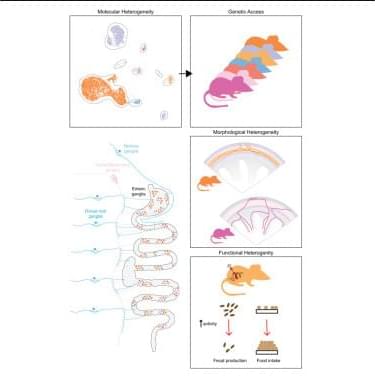
Brain development is a complex process involving, for example, the precise diversification and distribution of cells into distinct areas. The researchers behind the present study have developed a new method called spatial tri-omics, that enables them to simultaneously measure in a specific area of the brain: 1) the activity of genes, 2) how this activity is regulated by epigenetic changes, and 3) if this activity ultimately leads to the production of proteins.
The study is based on analyses of mouse and human brains at different stages of development. The authors generated a spatiotemporal tri-omic atlas of the mouse brain from postnatal day 0 (P0) to P21 and compared corresponding regions with the human developing brain.
“We’ve been able to use this multidimensional method to track brain development over time and map changes from birth to a young age in different parts of the brain, as well as study how the brain reacts to inflammation,” explains the senior author.