This is older but this is just the tip of the iceberg. China is rumored to be working on genetic engineering to create “super soldiers” and they’re one country that isn’t stopped by ethics concerns. In the Prime TV series “The peripheral” it has something similar and I don’t want to spoil it beyond that. I think there’s a Vin Diesel movie called Blood Shot where he’s made into a super soldier. It’s a shame that this is used for warfare but the plus side is it’ll, some of the tech, will make its way down to civilian life such as the Internet did.
Category: genetics – Page 228

Genome editing prevents hypertrophic cardiomyopathy in mice
In a recent study published in the journal Nature Medicine, researchers pursued one-time cures for hypertrophic cardiomyopathy (HCM). They used a previously constructed murine model of HCM, designated as R403Q-129SvEv, to evaluate two different genetic therapies, as follows:
I) an adenine base editor (ABE8e)
Ii) a potent Cas9 nuclease delivered by an adeno-associated virus (AAV) vector.
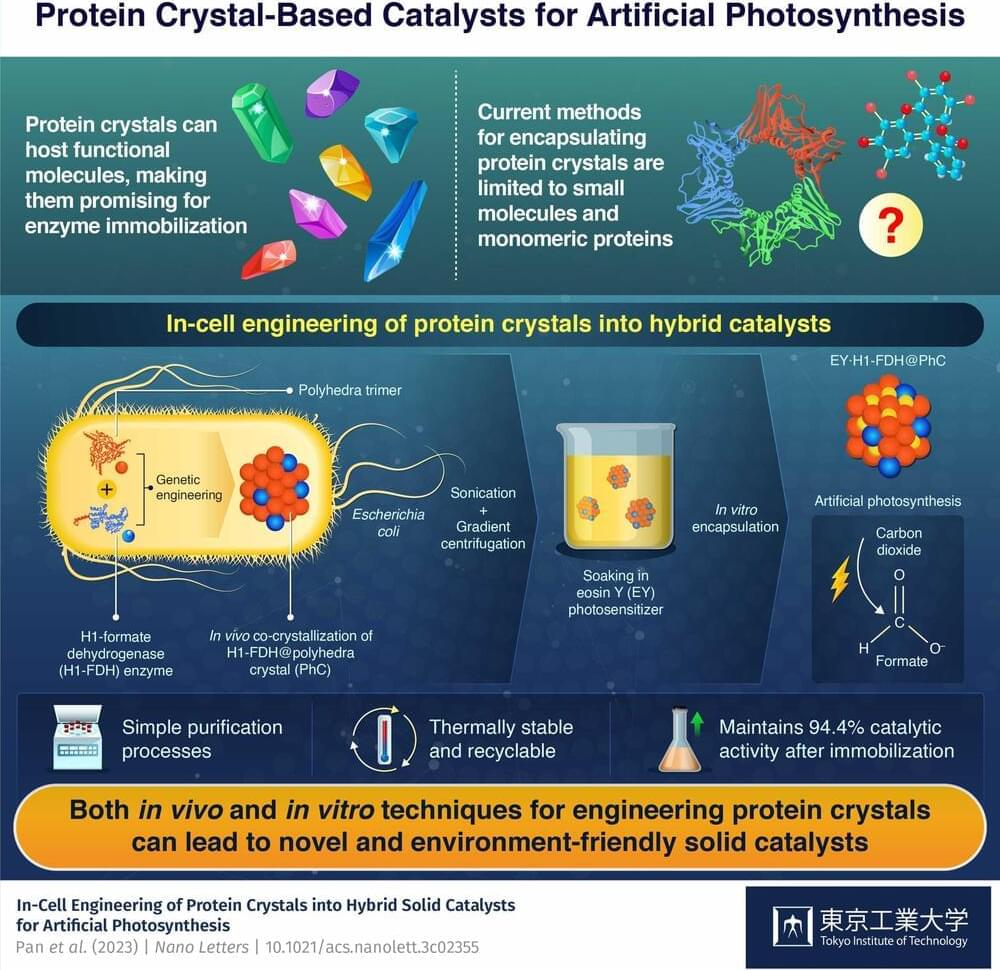
Artificial photosynthesis with engineering of protein crystals in bacteria
In-cell engineering can be a powerful tool for synthesizing functional protein crystals with promising catalytic properties, show researchers at Tokyo Tech. Using genetically modified bacteria as an environmentally friendly synthesis platform, the researchers produced hybrid solid catalysts for artificial photosynthesis. These catalysts exhibit high activity, stability, and durability, highlighting the potential of the proposed innovative approach.
Protein crystals, like regular crystals, are well-ordered molecular structures with diverse properties and a huge potential for customization. They can assemble naturally from materials found within cells, which not only greatly reduces the synthesis costs but also lessens their environmental impact.
Although protein crystals are promising as catalysts because they can host various functional molecules, current techniques only enable the attachment of small molecules and simple proteins. Thus, it is imperative to find ways to produce protein crystals bearing both natural enzymes and synthetic functional molecules to tap their full potential for enzyme immobilization.
How a BBQ lighter can make DNA vaccines more powerful
Georgia Tech researchers have transformed a standard BBQ lighter into a delivery system that uses an electric spark to boost DNA vaccines — and it could help increase global access to a cheap, powerful new vaccine technology.
mRNA vs. DNA vaccines: DNA vaccines deliver a bit of genetic code that tells cells in the body to make a protein from a specific virus or bacteria. That triggers the immune system to create antibodies against that protein that will protect you if you’re ever infected by that particular pathogen.
This is exactly how mRNA vaccines work, too, and just like mRNA vaccines, DNA-based shots are relatively cheap to produce and easy to change to make new vaccines — but the way mRNA and DNA vaccines get their genetic instructions into cells is different.
How Much Junk Food Is Bad For Health?
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links:
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
Use Code: ConquerAging At Checkout.
Epigenetic Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
At-Home Blood Testing: https://getquantify.io/mlustgarten.
Oral Microbiome: https://www.bristlehealth.com/?ref=michaellustgarten.
The First Step to Life: Hitting Reset To Start a New Embryo
Recent collaborative research conducted by scientists in the United States and China unveils the mechanism through which a fertilized egg cell, also known as a zygote, triggers a ‘reset’, enabling the newly formed embryo can develop according to its own genetic program. The study was recently published in the journal Nature.
It has been known for some time that the genome of a newly fertilized egg cell is inactive and has to be woken up, said Richard Schultz, research professor at the University of California, Davis, School of Veterinary Medicine and a corresponding author on the paper. This step is called zygote genome activation.
“For the embryo to develop, the oocyte/egg has to lose its identity and does so by making new stuff,” Schultz said. “We now know the first steps in how this transition occurs.”

Unearthing Our Past, Predicting Our Future: Scientists Discover the Genes That Shape Our Bones
This groundbreaking study, which was published as the cover article in the journal Science, not only sheds light on our evolutionary history but also paves the way for a future where physicians could more accurately assess a patient’s likelihood of suffering from ailments like back pain or arthritis later in life.
“Our research is a powerful demonstration of the impact of AI in medicine, particularly when it comes to analyzing and quantifying imaging data, as well as integrating this information with health records and genetics rapidly and at large scale,” said Vagheesh Narasimhan, an assistant professor of integrative biology as well as statistics and data science, who led the multidisciplinary team of researchers, to provide the genetic map of skeletal proportions.