Introduction: The lips fulfill various critical physiological roles besides being viewed as a fundamental aesthetic feature contributing to the perception of health and beauty. Therefore, any lip injury, abnormality, or congenital malformation, such as cleft lip, needs special attention in order to restore proper lip function and aesthetics. To achieve this goal, a better understanding of the complex lip anatomy, function, and biology is required, which can only be provided by basic research endeavors. However, the current lack of clinically relevant human lip cells and three-dimensional in vitro lip models, capable of replacing ethically questionable animal experimentations, represents a significant limitation in this area of research.
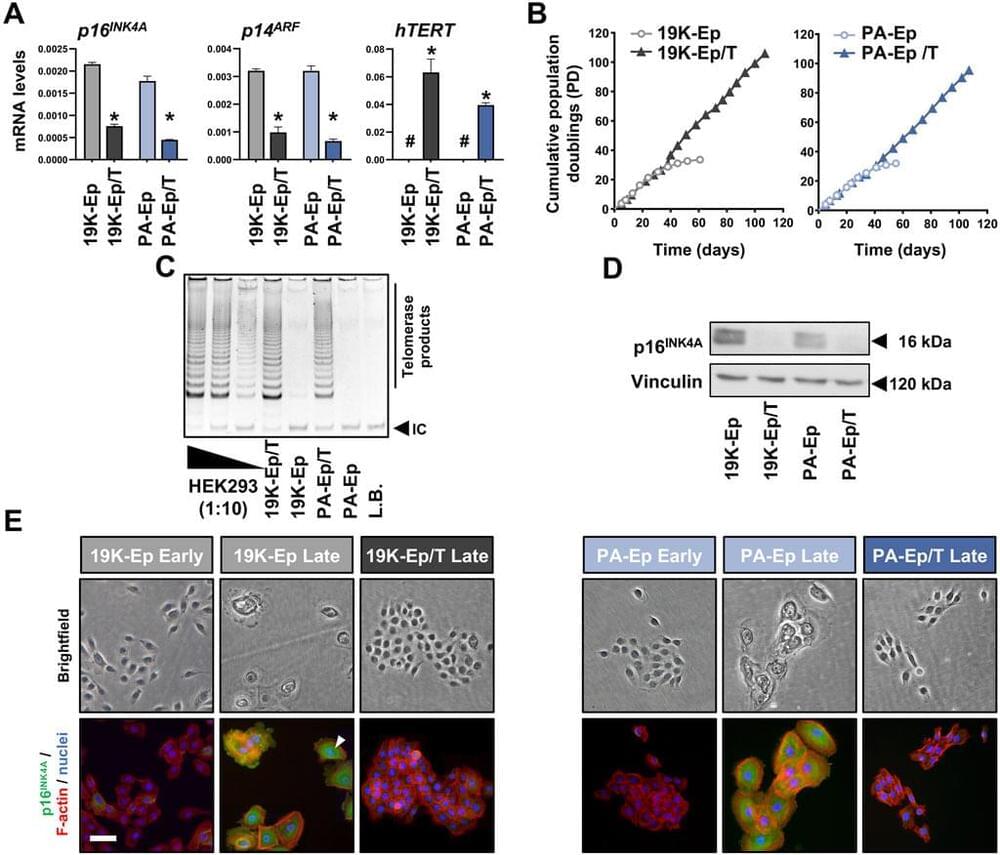
Methods: To address these limitations, we aimed to pioneer the introduction of immortalized healthy lip-and cleft lip-derived keratinocytes. Primary keratinocytes were isolated from patients’ samples and immortalized by introducing the catalytic domain of telomerase, combined with the targeted knockdown of the cell cycle inhibitor gene, p16INK4A. We then focused on validating the newly established cell lines by comparing their genetic stability and key phenotypic features with their primary keratinocyte counterparts.
Results: The newly established immortalized keratinocyte cell lines demonstrated genetic stability and preserved the main phenotypic characteristics of primary keratinocytes, such as cellular morphology and differentiation capacity. Three-dimensional lip models, generated using these cell lines, proved to be effective and convenient platforms for screening applications, including wound healing and microbial infection of the lip epithelium.