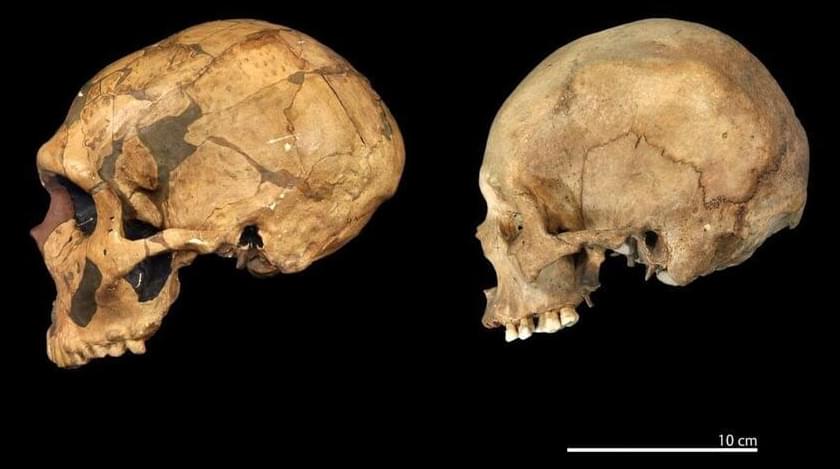
A study suggests that by the time H. sapiens expanded, the differentiation between the two species had progressed to the extent that they were distinct and recognizable as separate species.
A recent study conducted by researchers from London’s Natural History Museum and the Institute of Philosophy at KU Leuven has strengthened the argument that Neanderthals and modern humans (Homo sapiens) should be classified as distinct species to more accurately trace our evolutionary history.
Different researchers have different definitions as to what classifies as a species. It is undisputed that H. sapiens and Neanderthals originate from the same parental species, however studies into Neanderthal genetics and evolution have reignited the debate over whether they should be classed as separate from H. sapiens or rather a subspecies (H. sapiens neanderthalensis).