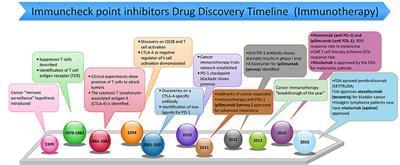
Year 2017 PD-1 can eventually be used throughout the body to be used on all cancers which also naturally occurs in the body the common cancer drug Keytruda uses this type of targeting to destroy cancer cells.
Several cancers are highly refractory to conventional chemotherapy. The survival of tumors in several cases is assisted by checkpoint immunomodulation to maintain the imbalance between immune surveillance and cancer cell proliferation. Check point antibody inhibitors, such as anti-PD-1/PD-L1, are a novel class of inhibitors that function as a tumor suppressing factor via modulation of immune cell-tumor cell interaction. These checkpoint blockers are rapidly becoming a highly promising cancer therapeutic approach that yields remarkable antitumor responses with limited side effects. In recent times, more than four check point antibody inhibitors have been commercialized for targeting PD-1, PDL-1, and CTLA-4. Despite the huge success and efficacy of the anti-PD therapy response, it is limited to specific types of cancers, which attributes to the insufficient and heterogeneous expression of PD-1 in the tumor microenvironment. Herein, we review the current landscape of the PD-1/PD-L1 mechanistic role in tumor immune evasion and therapeutic outcome for cancer treatment. We also review the current progress in clinical trials, combination of drug therapy with immunotherapy, safety, and future of check point inhibitors for multiple types of cancer.
Immunotherapy is an exciting approach, and tremendous strides have recently been made in our perception of the role of the host immune response in affecting tumor growth and response to various therapies (Pardoll, 2012). Through these advances, novel immune check point inhibitors have been identified and cleared for use in the clinic (Figure 1). The evolution of immune checkpoint inhibitors as anticancer treatment options represents one of the most successful approach in cancer drug discovery in the past few years (Couzin-Frankel, 2013). Indeed, immune checkpoint inhibitors have emerged as a frontline treatment for multiple cancers, such as metastatic melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCCs), and bladder or urothelial cancer. They are presently being assessed in numerous other cancer types, including breast cancer, head and neck cancer, and some advanced solid and hematological malignancies.