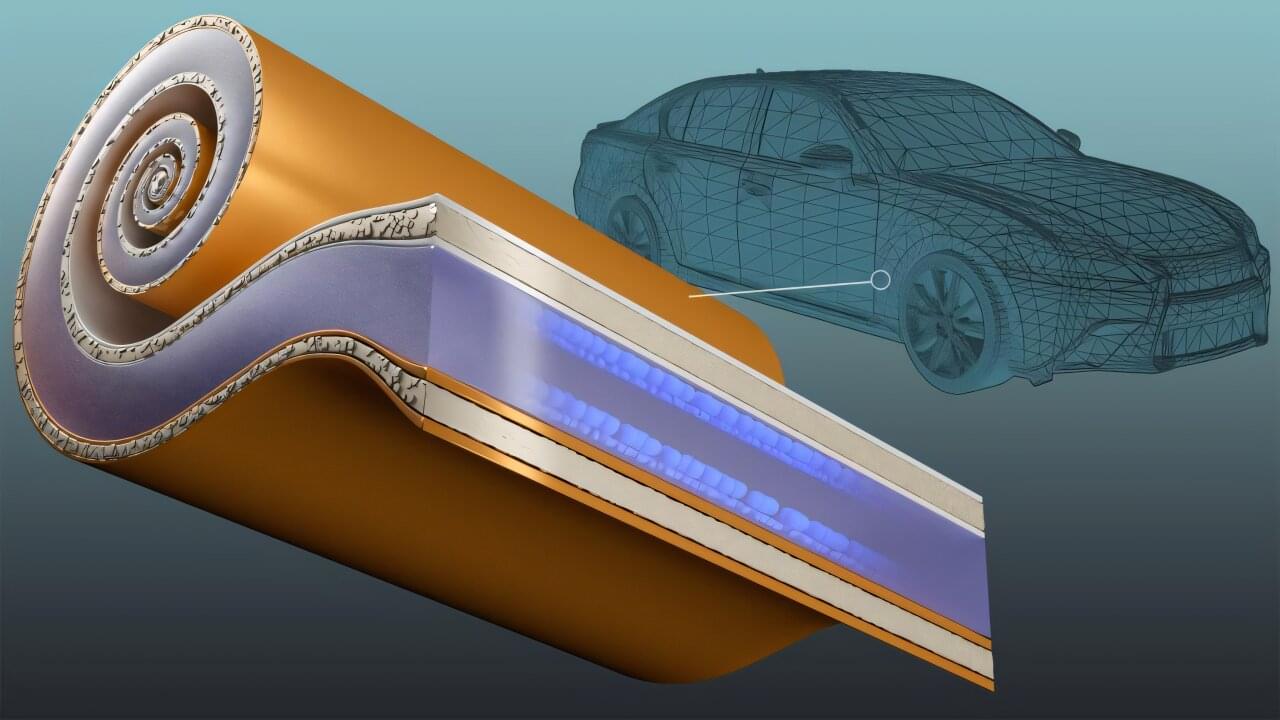
Strengthening the competitiveness of the American transportation industry relies on developing domestically produced electric vehicle batteries that enable rapid charging and long-range performance. The energy density needed to extend driving distance can, however, come at the expense of charging rates and battery life.
By integrating a new type of current collector, which is a key battery component, researchers at the Department of Energy’s Oak Ridge National Laboratory have demonstrated how to manufacture a battery with both superior energy density and a lasting ability to handle extreme fast charging. This enables restoring at least 80% of battery energy in 10 minutes. By using less metal, particularly high-demand copper, the technology also relieves strain on U.S. supply chains.
“This provides a significant savings on near-critical materials, because much less copper and aluminum are needed,” said lead researcher Georgios Polyzos. “At the same time, this will greatly enhance the energy density achievable with a 10-minute charge.”