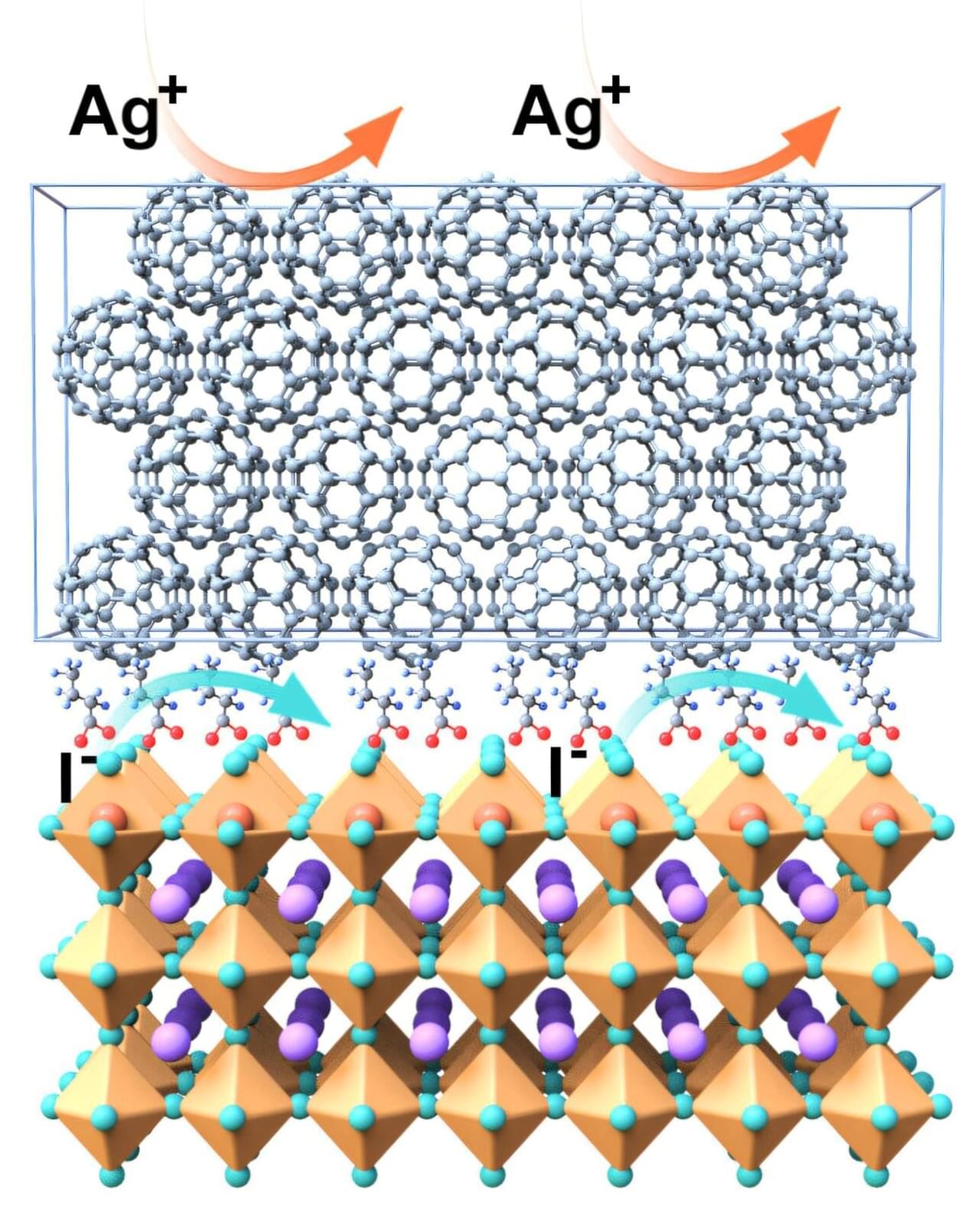
Perovskite solar cells are inexpensive to produce and generate a high amount of electric power per surface area. However, they are not yet stable enough, losing efficiency more rapidly than the silicon market standard. Now, an international team led by Prof. Dr. Antonio Abate has dramatically increased their stability by applying a novel coating to the interface between the surface of the perovskite and the top contact layer. This has even boosted efficiency to almost 27%, which represents the state-of-the-art.
After 1,200 hours of continuous operation under standard illumination, no decrease in efficiency was observed. The study involved research teams from China, Italy, Switzerland and Germany and has been published in Nature Photonics.
“We used a fluorinated compound that can slide between the perovskite and the buckyball (C60) contact layer, forming an almost compact monomolecular film,” explains Abate. These Teflon-like molecular layer chemically isolate the perovskite layer from the contact layer, resulting in fewer defects and losses. Additionally, the intermediate layer increases the structural stability of both adjacent layers, particularly the C60 layer, making it more uniform and compact.